Nature’s dyes
Natural dyes play essential roles in the animal kingdom. Beyond their aesthetic appeal, these compounds are biochemically significant and play key roles in cellular functions. One of the major organic-based classes of compounds responsible for pigments in nature is pterins. Pterins stand out not only for their vivid hues but also for their significance in the biochemistry of humans.1 Importantly, our deepening understanding of pterin pathways has led to medicinal breakthroughs, such as the development of an important class of chemotherapeutics, and has the potential to inspire new therapeutic strategies targeting cancer, neurological diseases, and immune disorders.2,3
The discovery of pterins
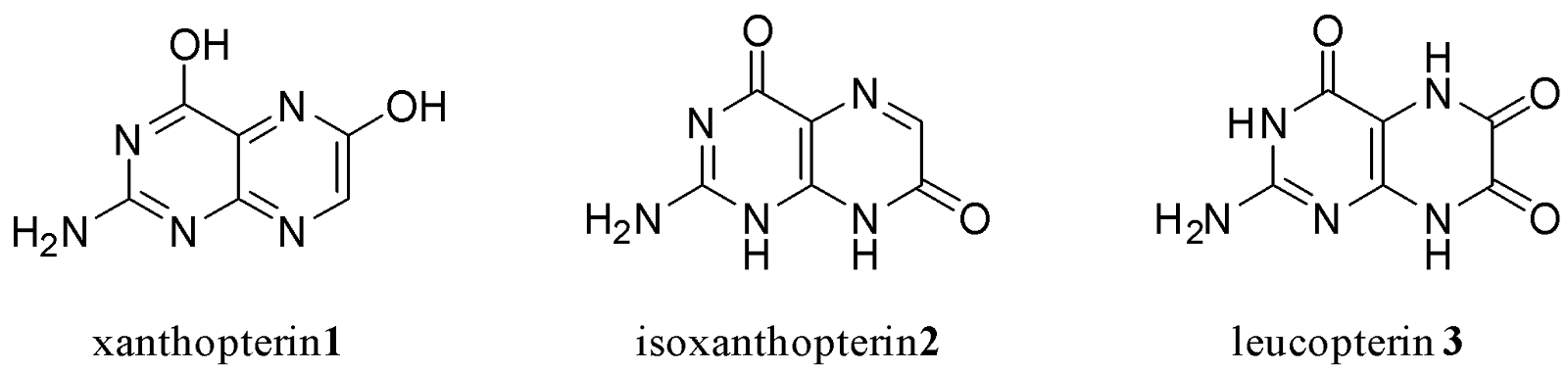
Pterins were first discovered in 1889 by Frederick Gowland Hopkins, who isolated a yellow pigment from the wings of the English brimstone butterfly.1,4 The pigment was appropriately named pterin, drawing from the Greek pteron, meaning “wing”.5 In later years, similar pigments were found in other insects, including tropical butterflies; however, the exact structure of these pterins remained unknown due to their unusual properties.6,7 Scientists found that these pterins could not be investigated using the analytical method of elemental analysis due to incomplete combustion, nor could the pigments be solubilised in common organic solvents. It wasn’t until 1940 that Purrman successfully elucidated the structure of the first pterins: xanthopterin 1, isoxanthopterin 2, and leucopterin 3 (Fig. 1).8
Naturally occurring pterins are highly conserved between species and have since been isolated from insects, birds, mammals, fish, amphibians, and plants, and range in pigmentation from white to yellow to red.9 As well as butterflies, pterins such as sepiapterin have been found in insects, such as the yellow pigment of a pillow bug, Armadillidium vulgare.9 Erythropterin and violapterin are responsible for the orange and red colouration on the European firebug, Pyrrhocoris apterus (Fig. 2a).9 The Mexican leaf frog, Agalychnis dacnicolor (Fig. 2b), contains the pterorhodin pigment, and the emperor penguin, Aptenodytes forsteri (Fig. 2c), contains the spheniscin pigment.9

These discoveries have yielded a large library of naturally occurring pterins which are one of the major sources of bright colouration in animals. However, pterins have far more biological significance than pigmentation, as they have crucial roles in cellular function.
The biosynthesis and chemistry of pterins
The pterin biosynthetic pathway
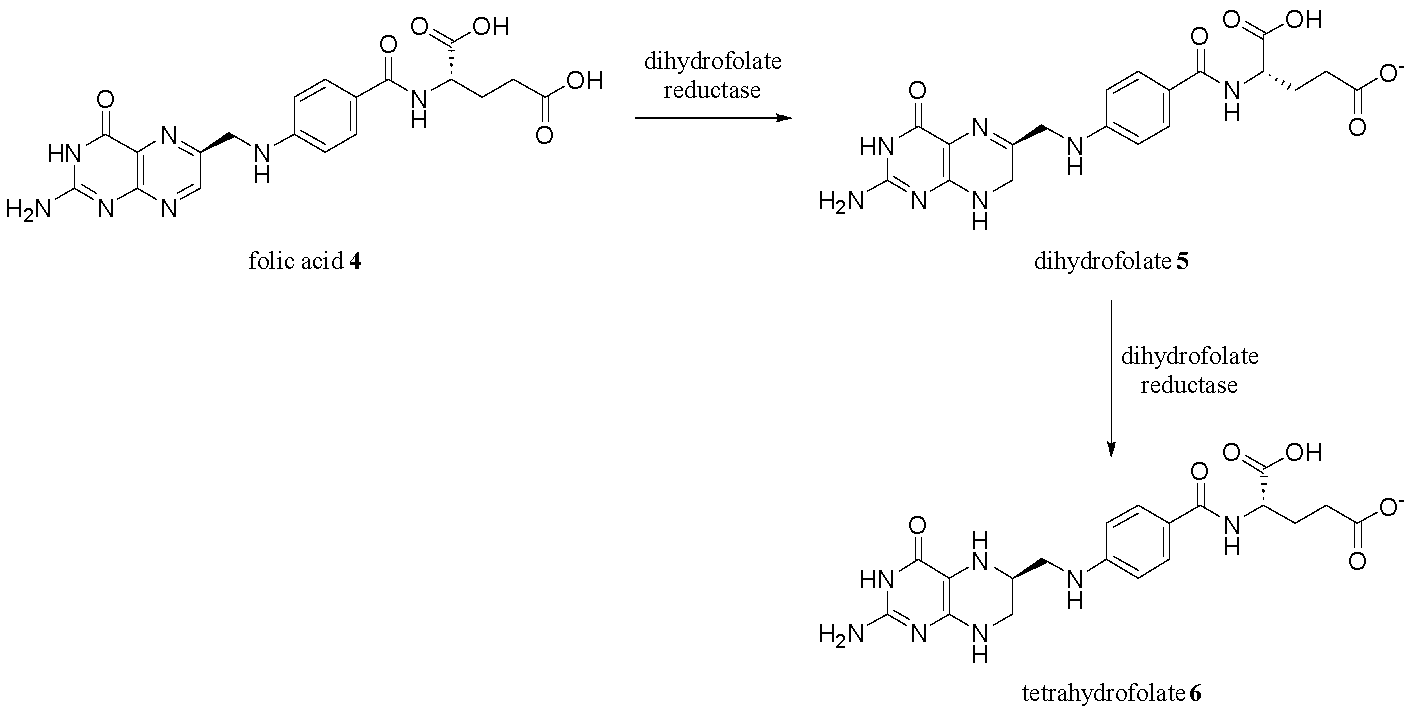
Perhaps the most well-known pterin-based compound in living organisms is folic acid (vitamin B9) 4.10 Humans cannot biosynthesise folic acid 4, so it is an essential nutrient that humans need to consume via fortified foods or dietary supplements.10 Once inside the body, folic acid 4 is reduced to dihydrofolate (DHF) 5 and then tetrahydrofolate (THF) 6 by the dihydrofolate reductase enzyme (Fig. 3). THF (6) is the active form of folic acid, which is the enzyme responsible for “delivering” an activated methyl group to a substrate in the biosynthesis of amino acids and nucleic acids.11
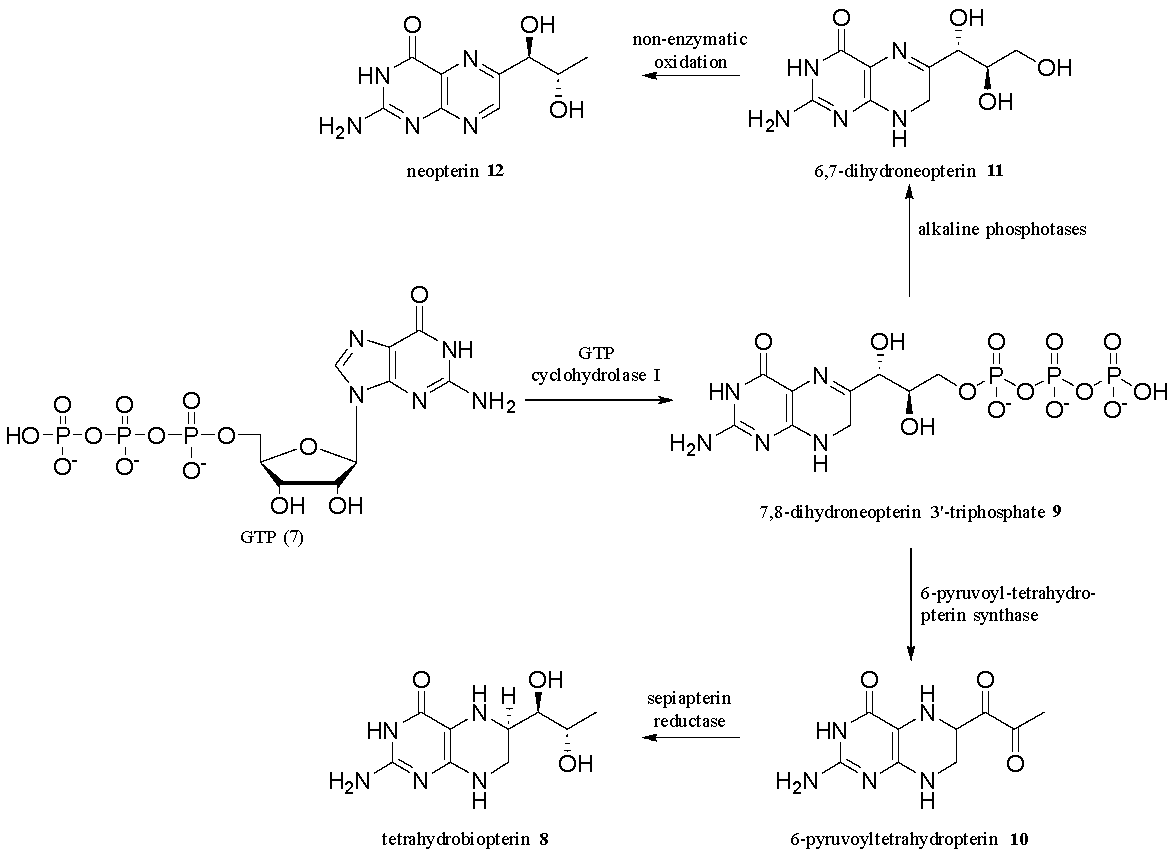
Although humans cannot produce folic acid, simpler pterin-based compounds can be produced endogenously (inside the cells of an organism) by the de novo rearrangement of guanosine.12 These pterin-based compounds are derived from guanosine triphosphate (GTP) via a series of enzyme-driven steps in which GTP (7) is converted to tetrahydrobiopterin 8 (Fig. 4). In the first step, GTP cyclohydrolase converts GTP 7 to 7,8-dihydroneopterin-3’-triphosphate 9. Phosphorylated neopterin (9) is converted to 6-pyruvoyltetrahydroneopterin 10 with the 6-pyruvoyltetrahydropterin synthase enzyme. Finally, sepiapterin reductase reduces the pyruvoylpterin (10) to tetrahydrobiopterin 8. Additional enzymatic transformations produce structurally diverse pterins with distinct physiological roles. For example, phosphorylated neopterin (9) can be converted to 6,7-dihydroneopterin 11 with alkaline phosphatases, then oxidised non-enzymatically to give neopterin 12.
Structure
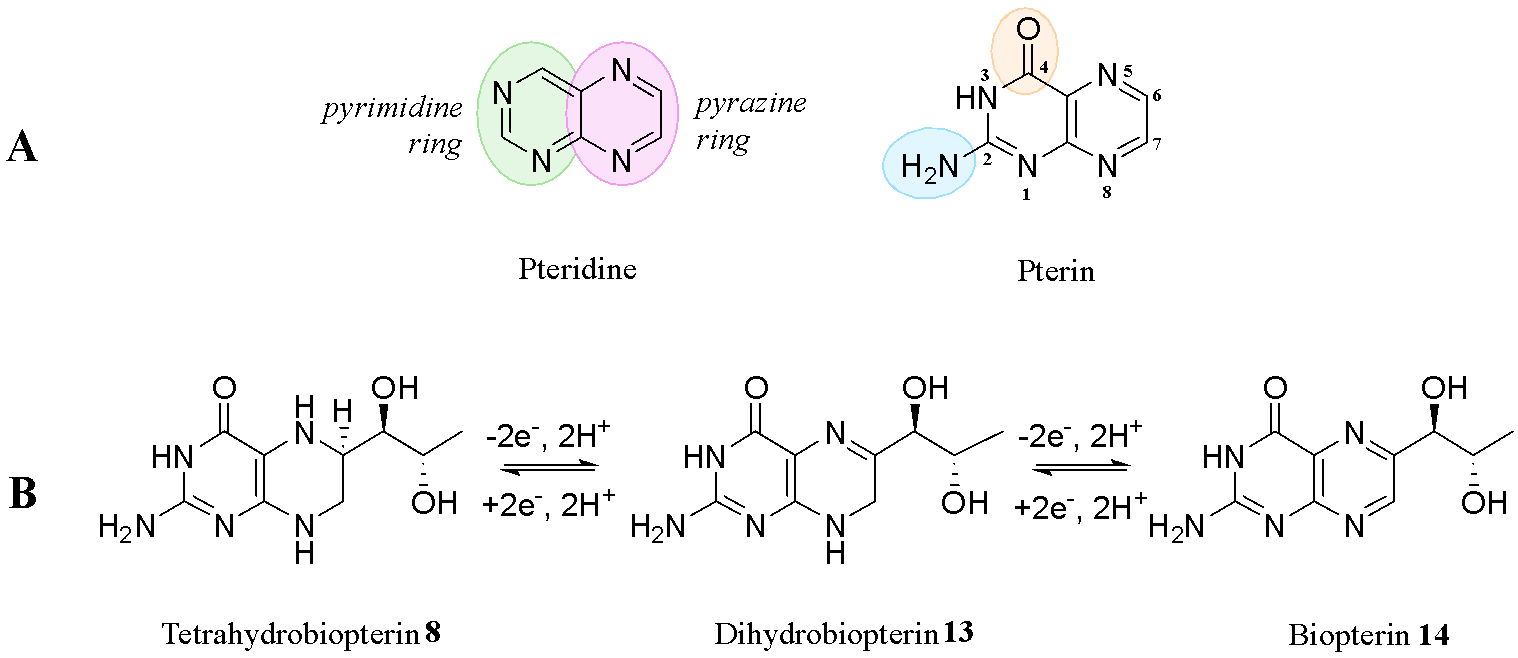
Pterins belong to the broader class of pteridines, which are bicyclic nitrogen heterocycles comprising a fused pyrazine and pyrimidine ring system (Fig. 5a). The defining features of pterins include an amino group at position 2 and a carbonyl group at position 4. These structural features confer reactivity relevant to both redox chemistry and tautomeric equilibria.
Redox states
Pterins can exist in three distinct redox states, each corresponding to the hydrogenation level of the pyrazine ring and are named accordingly. Tetrahydrobiopterin 8, for example, has the prefix tetrahydro, because it has four hydrogens (and no double bonds) on the pyrazine ring (Fig. 5b). Tetrahydrobiopterin 8 can be oxidised by taking away two hydrogens and two electrons and introducing a double bond to give dihydrobiopterin 13. Similarly, dihydrobiopterin 13 can be further oxidised to give biopterin 14. These transformations can be accomplished synthetically, as well as by enzymes such as sepiapterin reductase in the final step of the biosynthetic pathway of tetrahydrobiopterin 8, as seen in Fig. 4.
Tautomerisation
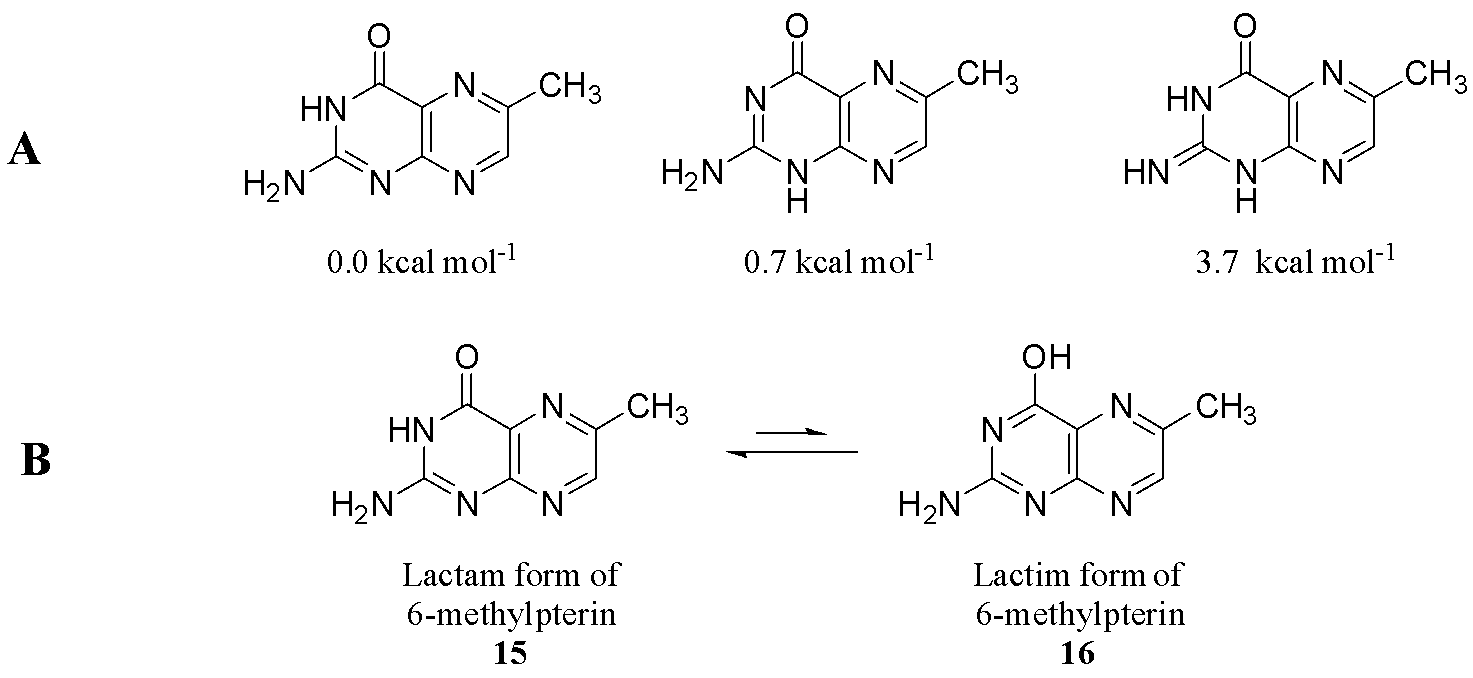
Fully oxidized pterins are conjugated and exhibit aromaticity, although the presence of four nitrogen atoms in the ring system disrupts uniform electron delocalization, rendering the molecule susceptible to nucleophilic attack. Pterins are capable of existing in at least three different interconvertible structures called tautomers. Density functional theory (DFT), a computational modelling method, was used to study the tautomers of 6-methylpterin (15). Pterin (15) can “rearrange” between three tautomers that were calculated to have a relative energy of less than 4 kcal mol-1 (Fig. 6a). In these tautomers, the arrangement of the double bond in the pyrimidine ring are different while the pyrazine ring remains conjugated.2
The fully oxidised pterin ring system can also tautomerise between the lactam (15) and lactim (16) forms (Fig. 6b). The lactam (15) form has a proton residing on nitrogen 3 while the lactim (16) form has a fully conjugated ring and the proton residing on the oxygen. DFT calculations of 6-methylpterin (15) suggest that this interconversion requires the exchange of protons between two molecules of water to achieve the lowest activation energy for tatuomerisation between the two states, as opposed to a simple proton shift.13 It is thought that the lactam form (15) is the more thermodynamically favourable form due to the tendency of the hydroxyl in the lactim (16) to oxidise free radicals.14
Biological relevance
As previously mentioned, folic acid is essential for cell growth and development. Because cancer cells as well as certain bacteria and viruses rely on rapid DNA synthesis, they are especially dependent on folate metabolism. This dependency has led to the development of antifolates - molecules that competitively inhibit dihydrofolate reductase (DHFR), thereby preventing the conversion of DHF 6 to THF 6 (as seen in Fig. 3).15 Methotrexate 17, one of the most successful antifolates, mimics DHF 6 structurally but lacks its biological function, effectively antagonising folate-dependent biosynthetic processes (Fig. 7).
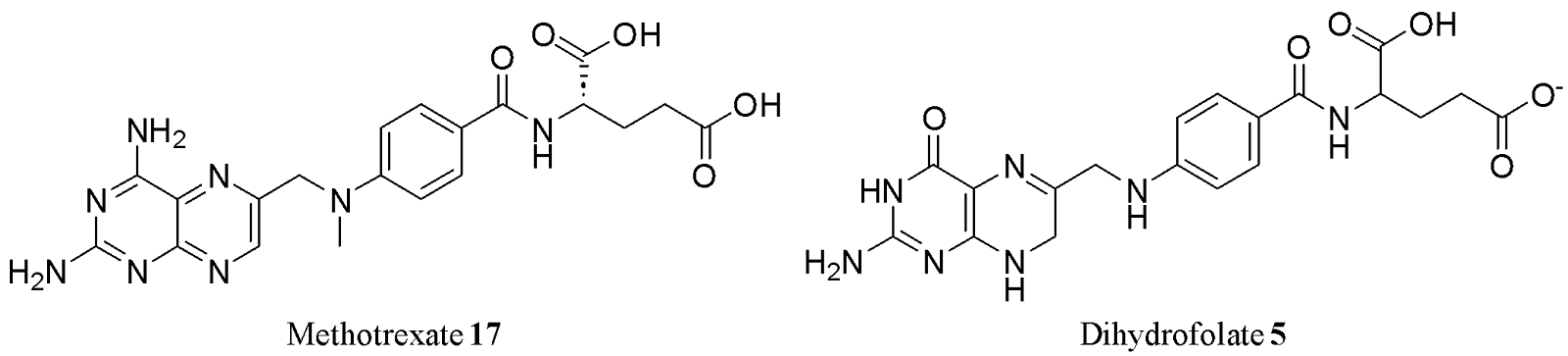
Methotrexate 17 was first used as a successful cancer chemotherapeutic for choriocarcinoma (cancer of the uterus) in 1961 and has since been used to treat several other types of cancer, as well as rheumatoid arthritis, improving patient outcomes dramatically.3 The success story of methotrexate 17 highlights other pterin derivatives of potential medicinal importance.
Pterins as redox cofactors
Beyond folate, other endogenously produced pterins have also shown significant clinical relevance. Tetrahydrobiopterin 8 (as seen in Fig. 4) is fully reduced and is an important enzymatic cofactor, meaning it acts as a “helper molecule” to aid enzymes in their role as catalysts to produce nitric oxide and break down amino acids like phenylalanine, tyrosine, and tryptophan.16 These are key steps in the production of important neurotransmitters such as serotonin and dopamine.16 Consequently, deficiencies in tetrahydrobiopterin are linked to diseases such as Parkinson’s disease,17 schizophrenia18 and Alzheimer’s disease.19
Non-redox active pterins as biomarkers
As oxidised pterins are the oxidation products of tetra- and dihydropterins, they are an effective marker of oxidative stress20, activation of the immune system21, cardiovascular diseases22 and cancer.23 Neopterin 12 is released in response to macrophage activation and can be measured to indicate the presence of autoimmune diseases, as well as viral and bacterial infections, including COVID-19.24,25 Another fully oxidised pterin in its lactim form, xanthopterin 1, has been found in high levels in patients with liver disease and hemolysis.26
Conclusions
Pterins are not only natural pigments but are central to a range of critical biological processes. While first discovered as a vibrant pigment in butterfly wings, the essential roles of pterins in enzymatic reactions, redox chemistry, and cellular regulation are now evident. Pterins serve as enzyme cofactors, biomarkers of disease, and therapeutic targets from folate metabolism to neurotransmitter synthesis and immune response. The structural versatility of pterins allows for different redox states and tautomeric forms, further underlining their functional importance. Understanding pterins not only broadens our knowledge of natural biochemistry but also informs synthetic targets in the treatment of metabolic, neurological, and autoimmune conditions.
References
- Andrade, P.; Carneiro, M. Pterin-Based Pigmentation in Animals. Biol Lett 2021, 17 (8). https://doi.org/10.1098/RSBL.2021.0221.
- Buglak, A. A.; Kapitonova, M. A.; Vechtomova, Y. L.; Telegina, T. A. Insights into Molecular Structure of Pterins Suitable for Biomedical Applications. Int J Mol Sci 2022, 23 (23), 15222. https://doi.org/10.3390/IJMS232315222.
- Methotrexate, J. B.-; 2000, undefined. Methotrexate: Historical Aspects. SpringerJR BertinoMethotrexate, 2000•Springer.
- Hopkins, F. G. Pigment in Yellow Butterflies. Nature 1891, 45 (1157), 197–198. https://doi.org/10.1038/045197C0).
- XV. The Pigments of the Pieridæ: A Contribution to the Study of Excretory Substances Which Function in Ornament. Philosophical Transactions of the Royal Society of London. (B.) 1895, 186, 661–682. https://doi.org/10.1098/RSTB.1895.0015.
- Pfleiderer, W. Pteridine, XXIV1) Über Die Isolierung Und Struktur Des Orangeroten Schmetterlingspigmentes “Erythropterin”2). Chem Ber 1962, 95 (9), 2195–2204. https://doi.org/10.1002/CBER.19620950916.
- Wijnen, B.; Leertouwer, H. L.; Stavenga, D. G. Colors and Pterin Pigmentation of Pierid Butterfly Wings. J Insect Physiol 2007, 53 (12), 1206–1217. https://doi.org/10.1016/J.JINSPHYS.2007.06.016.
- Purrmann, R. Über Die Flügelpigmente Der Schmetterlinge. VII. Synthese Des Leukopterins Und Natur Des Guanopterins. Justus Liebigs Ann Chem 1940, 544 (1), 182–190. https://doi.org/10.1002/JLAC.19405440111.
- Andrade, P.; Carneiro, M. Pterin-Based Pigmentation in Animals. Biol Lett 2021, 17 (8). https://doi.org/10.1098/RSBL.2021.0221.
- Folate - Health Professional Fact Sheet. https://ods.od.nih.gov/factsheets/folate-HealthProfessional/ (accessed 2025-04-23).
- Basu, P.; Burgmayer, S. J. N. Pterin Chemistry and Its Relationship to the Molybdenum Cofactor. Coord Chem Rev 2011, 255 (9–10), 1016. https://doi.org/10.1016/J.CCR.2011.02.010.
- Basu, P.; Burgmayer, S. J. N. Pterin Chemistry and Its Relationship to the Molybdenum Cofactor. Coord Chem Rev 2011, 255 (9–10), 1016. https://doi.org/10.1016/J.CCR.2011.02.010.
- Yamabe, S.; Tsuchida, N.; Physical, S. Y.-T. J. of; 2021, undefined. How Is the Oxidation Related to the Tautomerization in Vitamin B9? The Journal of Physical Chemistry A, 2021•ACS Publications 2021, 125 (42), 9346–9354. https://doi.org/10.1021/acs.jpca.1c06678.
- Joshi, R.; Adhikari, S.; Patro, B. S.; Chattopadhyay, S.; Mukherjee, T. Free Radical Scavenging Behavior of Folic Acid: Evidence for Possible Antioxidant Activity. Free Radic Biol Med 2001, 30 (12), 1390–1399. https://doi.org/10.1016/S0891-5849(01)00543-3.
- Bailey, L. B.; Gregory, J. F. Folate Metabolism and Requirements. Journal of Nutrition 1999, 129 (4), 779–782. https://doi.org/10.1093/JN/129.4.779.
- Kappock, T. J.; Caradonna, J. P. Pterin-Dependent Amino Acid Hydroxylases. Chem Rev 1996, 96 (7), 2659–2756. https://doi.org/10.1021/CR9402034/ASSET/IMAGES/MEDIUM/CR9402034E00013.GIF.
- Kurosaki, H.; Yamaguchi, K.; Man-yoshi, K.; Muramatsu, S. ichi; Hara, S.; Ichinose, H. Administration of Tetrahydrobiopterin Restored the Decline of Dopamine in the Striatum Induced by an Acute Action of MPTP. Neurochem Int 2019, 125, 16–24. https://doi.org/10.1016/J.NEUINT.2019.02.005.
- Zhilyaeva, T. V.; Kasyanov, E. D.; Semennov, I. V.; Rukavishnikov, G. V.; Piatoikina, A. S.; Kostina, O. V.; Verbitskaya, E. V.; Mazo, G. E. Tetrahydrobiopterin Deficiency in Schizophrenia: Biochemical and Clinical Aspects. J Psychiatr Res 2022, 153, 141–148. https://doi.org/10.1016/J.JPSYCHIRES.2022.07.020.
- Fanet, H.; Tournissac, M.; Leclerc, M.; Caron, V.; Tremblay, C.; Vancassel, S.; Calon, F. Tetrahydrobiopterin Improves Recognition Memory in the Triple-Transgenic Mouse Model of Alzheimer’s Disease, Without Altering Amyloid-β and Tau Pathologies. Journal of Alzheimer’s Disease 2021, 79 (2), 709. https://doi.org/10.3233/JAD-200637.
- Wakabayashi, I.; Nakanishi, M.; Ohki, M.; Reports, A. S.-S.; 2020, undefined. A Simple and Useful Method for Evaluation of Oxidative Stress in Vivo by Spectrofluorometric Estimation of Urinary Pteridines. nature.comI Wakabayashi, M Nakanishi, M Ohki, A Suehiro, K UchidaScientific Reports, 2020•nature.com 2020, 10 (1), 11223. https://doi.org/10.1038/s41598-020-67681-4.
- Murr, C.; Widner, B.; Wirleitner, B.; Fuchs, D. Neopterin as a Marker for Immune System Activation. Curr Drug Metab 2005, 3 (2), 175–187. https://doi.org/10.2174/1389200024605082.
- Fuchs, D.; Avanzas, P.; Arroyo-Espliguero, R.; Jenny, M.; Consuegra-Sanchez, L.; Kaski, J. C. The Role of Neopterin in Atherogenesis and Cardiovascular Risk Assessment. Curr Med Chem 2009, 16 (35), 4644–4653. https://doi.org/10.2174/092986709789878247.
- Hausen, A.; Fuchs, D.; Reibnegger, G.; Cancer, H. W.-; 1984, undefined. Urinary Pteridines on Patients Suffering from Cancer. A Comment on the Method and Results of Rao and Associates and of Trehan and Associates. Wiley Online LibraryA Hausen, D Fuchs, G Reibnegger, H WachterCancer, 1984•Wiley Online Library. https://doi.org/10.1002/1097-0142(19840401)53:7<1634::AID-CNCR2820530736>3.0.CO;2-8.
- Dogheim, G. M.; Amralla, M. T.; Werida, R. H. Role of Neopterin as an Inflammatory Biomarker in Congestive Heart Failure with Insights on Effect of Drug Therapies on Its Level. Inflammopharmacology 2022, 30 (5), 1617. https://doi.org/10.1007/S10787-022-01028-5.
- Bellmann-Weiler, R.; Lanser, L.; Burkert, F.; Seiwald, S.; Fritsche, G.; Wildner, S.; Schroll, A.; Koppelstätter, S.; Kurz, K.; Griesmacher, A.; Weiss, G. Neopterin Predicts Disease Severity in Hospitalized Patients With COVID-19. Open Forum Infect Dis 2021, 8 (1). https://doi.org/10.1093/OFID/OFAA521.
- Qujeq, D.; Ahmadi, H. Determination of Xanthopterin in Patients with Renal Insufficiency. Am J Nephrol 2001, 21 (4), 340–342. https://doi.org/10.1159/000046271.