Introduction
Tuberculosis (TB) is an infectious disease that most commonly affects the lungs and is caused by the bacteria Mycobacterium tuberculosis (Mtb). TB is spread through the air by the expulsion of infectious aerosol droplets and the most common symptoms associated with pulmonary TB are a chronic cough, fever, night sweats and weight loss.1,2
It is believed that the emergence of Mtb as a human pathogen began some 70,000 years ago in Africa, originating from smooth tubercle bacilli in the environment. Mtb was initially unable to cause chronic infections in the immunocompetent or spread between humans. This was followed by a genetic bottleneck around 35,000 years ago, which led to Mtb strains that could survive in low density populations and reactivate after long periods of latency. As humans domesticated animals, the disease was passed onto livestock and the advancement of human civilisation, such as agriculture and migration to urban settings, allowed the evolution of Mtb strains into the modern, highly viral and transmissible strains known today.3–5
Approximately one third of the global population is infected with a latent form of the disease but only 10% of these cases will progress to active TB. Without proper treatment around half of people infected with TB will die. The disease disproportionally affects men more than women, although the exact reason for this disparity remains unknown.6,7 TB continues to be a major public health challenge in the 21st century, despite being both curable and preventable. TB was the leading cause of death from a single infectious agent in 2024, accounting for an estimated 1.25 million deaths.8
The World Health Organisation (WHO) estimated that 10.8 million people developed TB in 2024. It is still the most prevalent disease in low-income countries, where it is closely linked to the broader challenges of poverty and limited access to healthcare. Many of these countries face significant challenges in managing the TB burden, which contributes to difficulties in sustaining treatment adherence. This has also led to the rise of drug-resistant strains of TB, such as rifampicin resistant (RR), multidrug resistant (MDR), extensively drug resistant (XDR) and pre-XDR TB. There were an estimated 400,000 cases of drug-resistant TB in 2023, with 28,982 confirmed cases of pre-XDR/XDR TB.8
The biology of TB: why is it so hard to treat?
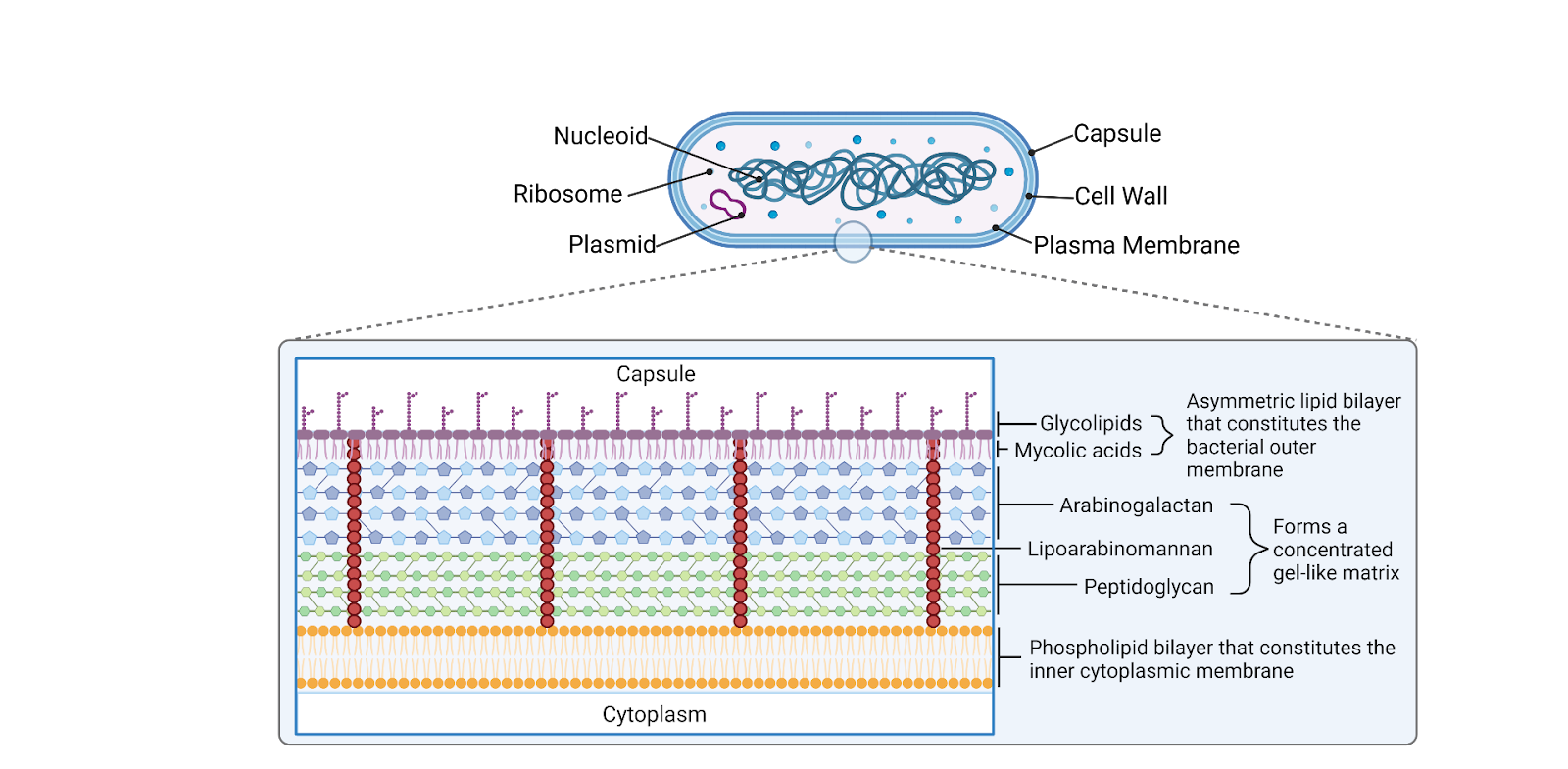
Mtb is a slowly growing bacterium, with its population doubling every 12-24 hours under optimal conditions. Its cell wall structure provides an extraordinarily strong impermeable barrier (Fig. 1), contributing to its resilience and resistance to many antibiotics.9 Infection is caused in new hosts when the bacilli reach the alveoli, where it is met with the host immune response. 5,10
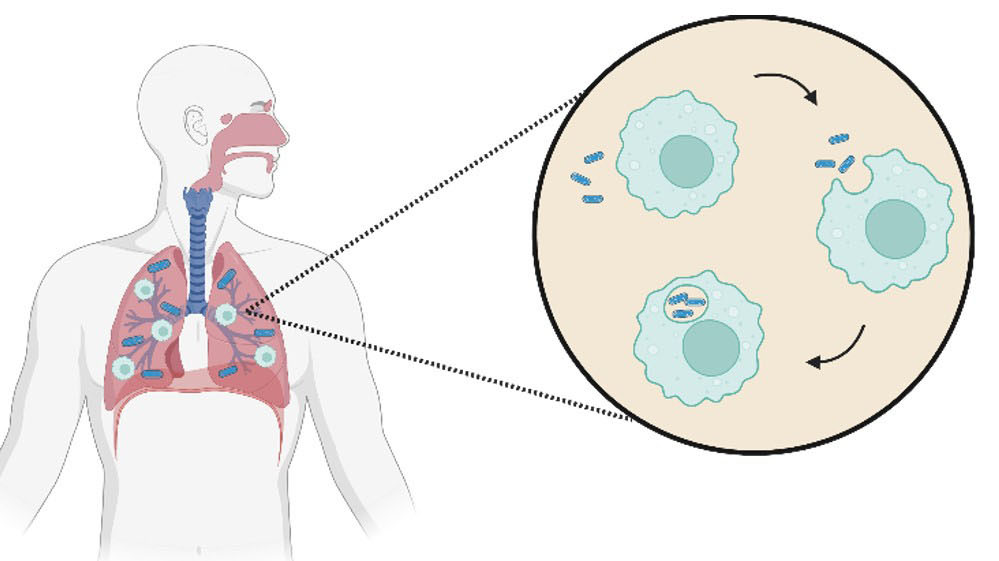
Alveolar macrophages engulf Mtb, and under normal circumstances, the invading bacteria would be digested by phagosome fusion with the lysosome. However, TB can actively block this fusion. If the host successfully eliminates the bacteria, either with an innate or acquired immune response, they will not contract TB.11
If elimination is unsuccessful, Mtb can start to replicate within the macrophages and diffuse to nearby cells where it will continue to spread, diffusing to other organs through the bloodstream. An adaptive immune response then kicks in, sending immune cells to the primary infection site. These form a granuloma, which walls off the infection and is then covered in fibrotic components that become calcified. This traps the invading bacteria and creates a Ghon complex. The exact process that occurs inside the granuloma remains unclear, however it is agreed that there must be replication of the bacteria occurring. The replicating bacilli would then be processed and eliminated by host. If the disease does not progress, the individual remains infected with latent TB (Fig. 2).5,9,12–17
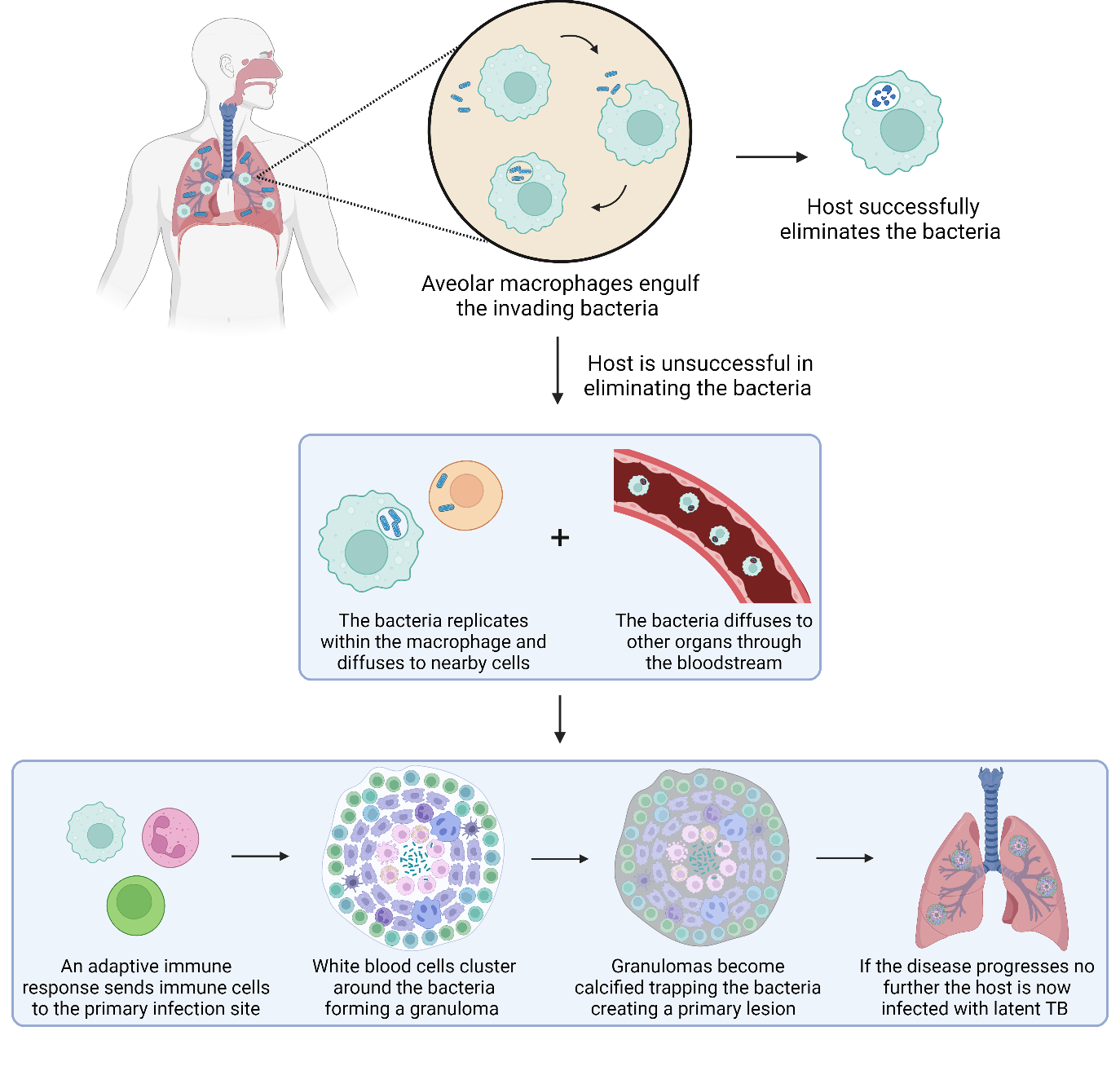
Around 10% of people infected with latent TB will progress to active TB. This can occur within months to years.18 Individuals with latent TB are not infectious and will not contribute to the spread of the disease. For reasons yet to be fully determined, the progression to active TB happens when the bacterial load becomes too high a burden and can no longer be contained. Mtb then re-enters the bloodstream and spreads throughout the body.19 The progression to active TB can be subtle with some asymptomatic patients still able to spread the disease, this is known as subclinical TB.20
Patients with active TB may have drug-susceptible or drug-resistant TB. Drug resistance can range from resistance to one of the first-line drugs to all of them, as well as some second-line drugs. Drug resistance makes TB increasingly more difficult to treat. Considering the majority of MDR cases are assumed to be from transmission and not initial acquisition, rapid detection and diagnoses are critical to help combat the spread.21–24
TB treatment arsenal
Preventative treatment
There is a high mortality rate for infants under five associated with TB, so in high-risk countries the Bacille Calmette-Guérin (BCG) vaccine is used as a preventative measure. However, the efficacy for pulmonary TB can vary drastically for adults. It is not fully understood why protective immunity is not sustained or why it fails to prevent primary infection, reactivation and active TB in some individuals but not others. BCG is the only approved vaccine for TB in humans and played a significant role in preventing a large number of deaths since it was introduced.25–27
Drug-susceptible and latent treatment
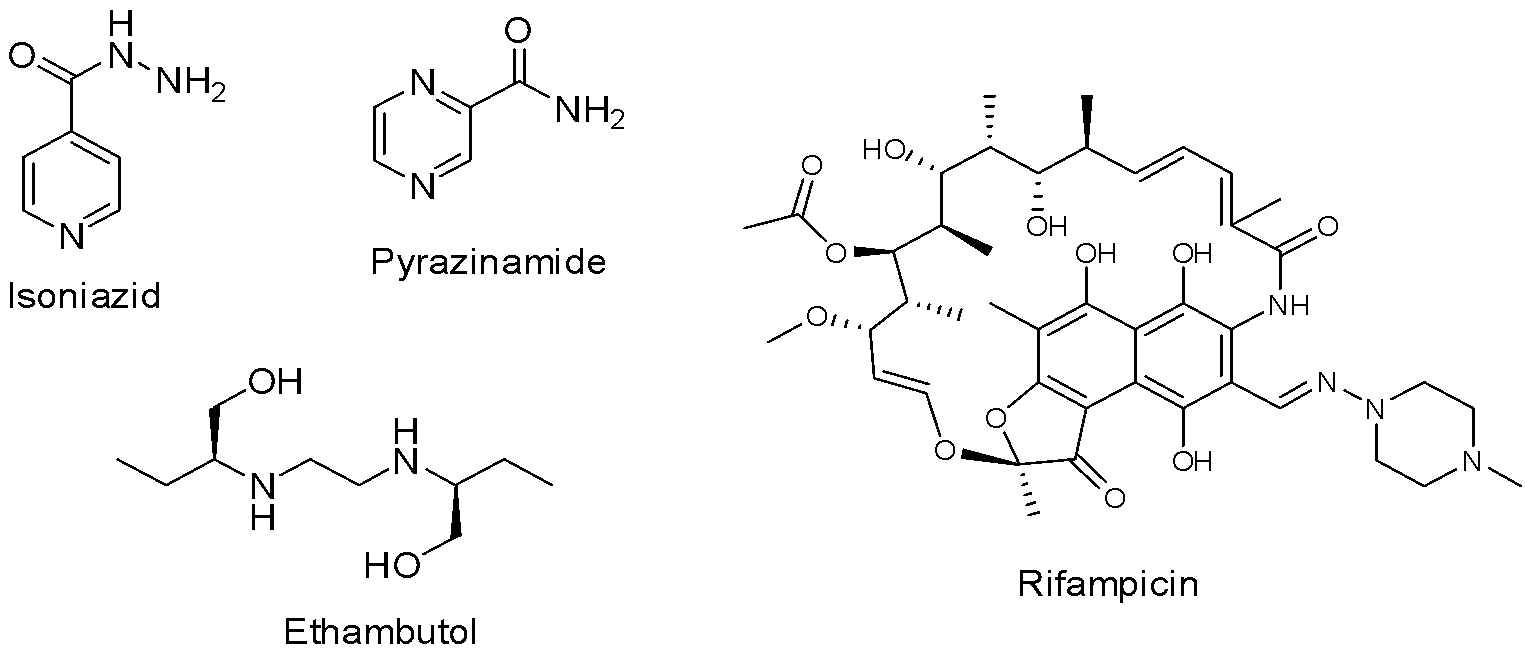
In the 1940s, streptomycin and p-aminosalicyclic acid were introduced as a treatment for TB. They were moderately effective but had significant side effects and suffered from the development of drug resistance when taken independently.28,29 In 1951, isoniazid (Fig. 3) was implemented as an anti-TB drug and it is still one of the most effective first-line drugs. Using isoniazid on its own led to patient relapse and drug resistance, which demonstrated the importance of multidrug therapeutic strategies. This ‘triple therapy’ took between 18-24 months to complete, consisting of oral isoniazid and p-aminosalicyclic acid for the entire regimen and intramuscular injections of streptomycin for the initial six months. This cured 90-95% of patients during trials in the 1950s. Ethambutol (Fig. 3) replaced p-aminosalicyclic acid in the 1960’s after it was shown to be effective on isoniazid and streptomycin-resistant strains.30,31
Following this, streptomycin was replaced with rifampicin (Fig. 3), allowing regimens to be shortened to nine months. Lastly, pyrazinamide (Fig. 3) was added to the multidrug therapy as it permitted the regimens to be shortened even further to six months. It requires patients to take a combination of isoniazid, rifampicin, pyrazinamide and ethambutol for the first two months, averaging ten pills a day, followed by four months of isoniazid and rifampicin. This course of treatment is both economically viable, costing around $20 USD, and highly successful, curing up to 85% of patients when administered under a strict program.32–34
More recently, attempts have been made to shorten the six-month regimen to only four in the hopes of increasing patient adherence. This was achieved by using rifapentine instead of rifampicin and moxifloxacin instead of ethambutol, in addition to pyrazinamide and isoniazid (Fig. 4). It proved to be as effective as the usual six-month regimen. However, moxifloxacin is currently used to combat drug-resistant TB so measures would be needed to ensure proper use and decrease the risk of developing antibiotic resistance. Furthermore, rifapentine is slightly more expensive and less readily available than rifampicin, making the short-term implementation of the regimen difficult.35–37
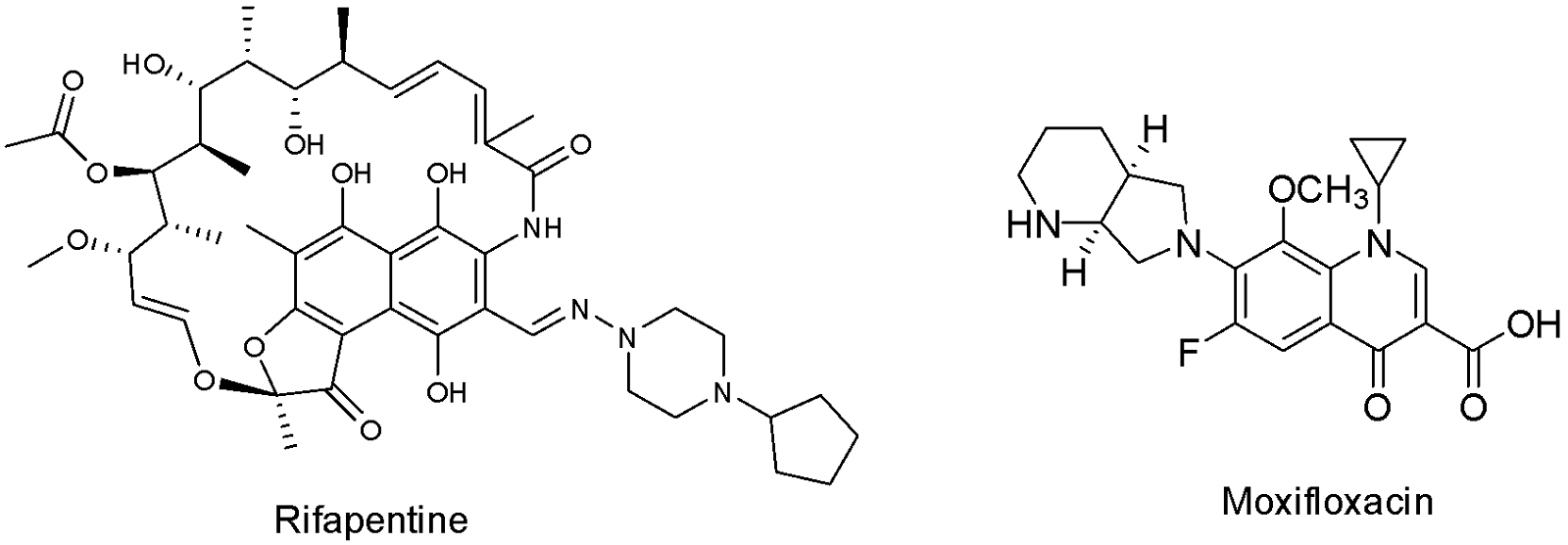
There are four recommended regimens for latent TB, and the course of treatment implemented depends on the individual. Each regimen consists of one or a combination of three drugs: isoniazid, rifapentine and rifampicin. While the first two regimens consist of isoniazid taken daily or semi-weekly for six to nine months, the third includes isoniazid and rifapentine taken weekly for three months. Finally, the fourth regimen is rifampicin taken daily for four months. Unfortunately, for patients with latent TB who have been exposed to drug-resistant strains, there are currently no officially recommended treatment regimens.38,39
Drug-resistant TB
Rifampicin-resistant (RR) TB and MDR TB do not respond to at least two of the most powerful first-line TB drugs, isoniazid and rifampicin. However, patients with either of these strains are often grouped together as they are both eligible for MDR TB treatment regimens. The length of the regimen and combination of first- and second-line treatment varies from patient to patient and depends on a number of factors such as resistance or intolerance. Treatment for MDR/RR TB lasts at least nine months but may require up to several years to be effective. The second-line drugs are made up of a number of different drugs, including fluoroquinolones.
Long-term MDR treatment regimens are categorised by WHO into three different groups, based on their effectiveness and toxicity.40
Group A: These drugs are the most highly recommended, unless the specific strain being treated is resistant or they are deemed unsuitable for the patient. It consists of levoflaxin, moxifloxacin, bedaquiline and linezolid (Fig. 5).
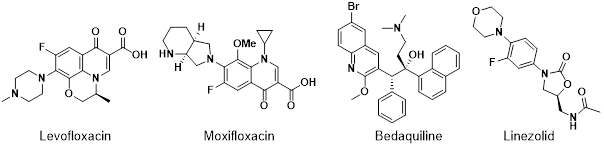
The fluoroquinolones, levoflaxin and moxifloxacin demonstrated efficacy against growing and non-growing Mtb. These drugs are used for the treatment of MDR TB because their mechanism of action is different to that of isoniazid and rifampicin. However, there are a few associated adverse effects, such as gastrointestinal problems, central nervous system issues and QT prolongation.41–43 Linezolid is an oxazolidinone antibiotic, and permanent discontinuation is slightly more frequent compared to fluoroquinolones. Side effects such as peripheral and ocular neuropathy, anaemia, thrombocytopenia and reticulocytopenia can occur.44 Bedaquiline, a diarylquinoline compound, can cause adverse side effects included peripheral neuropathy, gastrointestinal problems, and QT prolongation (a heart condition).45,46
Group B: These second line therapeutic agents tend to have more adverse side effects, be less readily available and less effective. Therefore, their use is more limited than that of group A. It consists of clofazimine, D-cycloserine and terizidone.
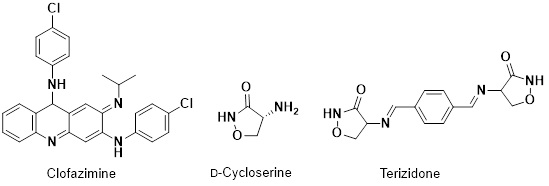
Clofazimine (Fig. 6) is a riminophenazine dye that is highly lipophilic. Side effects include gastrointestinal problems and an orange/pink or brownish/black discolouration of the skin, conjunctivae and bodily fluids, which can take up to several years to reverse.47 D-cycloserine is a structural analogue of D-alanine and has a number of neurological side effects such as psychosis, dysarthria, convulsions, depression and seizures.48–50 Terizidone is a derivative of D-cycloserine but fewer adverse events are reported.51
Group C: This group consists of a wide range of compounds (Fig. 7). Group C drugs generally have more adverse side-effects than groups A and B. They are utilised when the drugs from the other groups are unsuitable.
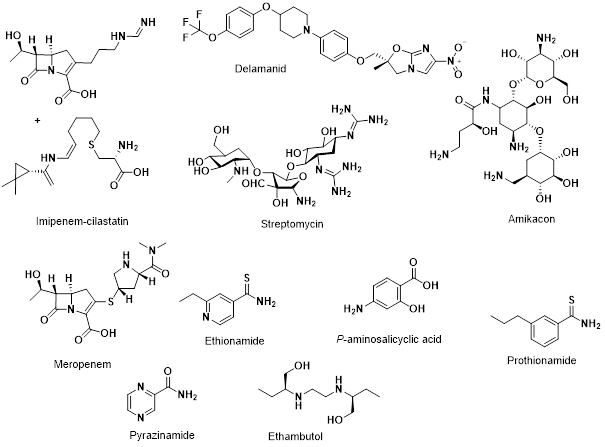
Delamanid is a relatively new drug, having only being conditionally approved for treatment of MDR TB in 2014. It is a dihydro-nitroimidazooxazole derivative. As there are less data available regarding the safety and associated side effects, delamanid is currently part of the group C. 52,53
Imipenem and meropenem are both carbapenems, a sub class of the β-lactam family of antibiotics. Imipenem is degraded by the enzyme dehydropeptidase and is therefore combined with cilastatin, a human dehydropeptidase inhibitor. Hydrolysis of the β-lactam ring is known to occur by broad-spectrum mycobacterial class A β-lactamase enzymes, limiting their use. To circumvent this, they are often used in conjunction with a β-lactamase inhibitor such as clavulanate. Side effects for imipenem-cilastatin includes nausea, vomiting, diarrhoea and headaches, with adverse effects such as sloughing of the skin, confusion and seizures. Meropenem has similar side effects with the addition of anaemia and yeast infections in the mouth or throat.54,55
Amikacin and streptomycin are aminoglycosides, and as previously mentioned, streptomycin was initially used as a treatment for drug-susceptible TB, until the emergence of streptomycin resistance. Amikacin was initially classified as a group B drug but due to its toxicity it was reclassified as group C. Both prolonged use and high dosage can cause nephrotoxicity, ototoxicity, vestibular toxicity and congenital deafness and so are classified as group C drugs.56–58 However, there has been some evidence suggesting that the use of streptomycin could be expanded, especially in countries where newer drugs are less readily available.59
Ethionamide and prothionamide are both thioamides, which are analogues of amides. Both are structurally similar to isoniazid and therefore share the same inhibition target. The side effects associated with ethionamide and prothionamide include gastrointestinal disturbances, endocrine disorders and dizziness with adverse events including hepatitis, depression and peripheral or optic neuropathy.60
P-aminosalicyclic acid was the second-line drug used to treat TB after the use of streptomycin. However, it was quickly replaced with the more potent and better tolerated isoniazid, pyrazinamide, rifampicin and ethambutol. Drug resistance has caused scientists to revert back to using p-aminosalicyclic acid for treatment, in the same way as streptomycin. Despite the fact that this drug has been in use for more than 70 years, its exact mechanism of action is still not fully understood.61–63 It can cause side effects including gastrointestinal disturbances, hypothyroidism, hepatitis and blood disorders.64
As of January 2021, XDR TB, is classified as any Mtb strain that fulfils the definition of either RR or MDR TB in addition to being resistant to any fluoroquinolone and at least one other of the group A second-line drugs. WHO defined pre-XDR TB as a new class to highlight its seriousness. Pre-XDR TB is defined as any TB that fulfils the definition of RR/MDR TB in addition to being resistant to any fluoroquinolone.65 There are no regimen recommendations for the treatment of XDR. As with treatment for MDR TB, there are many factors that influence the selection of drugs and the duration of treatment. In some cases of MDR/XDR TB, a surgical intervention is also considered, but the data limited as to whether this increases the chance of survival.40,66,67
Conclusions
Tuberculosis remains a significant global health challenge, particularly in the face of rising drug resistance. While effective treatment exists, the emergence of drug-resistant TB has complicated management and increased the burden on healthcare systems in countries already facing significant challenges.
Notably, the success rate for MDR TB patients is estimated to be only 59% and for XDR patients around 40%.68,69 There is a need for a comprehensive approach to effectively combat TB. One that not only includes new therapeutics using new mechanisms of action, but also focuses on equity to ensure a globally effective approach.
References and notes
- Extrapulmonary Tuberculosis https://www.msdmanuals.com/en-nz/professional/infectious-diseases/mycobacteria/extrapulmonary-tuberculosis-tb (accessed 29/03/2022).
- Sanford, C. A.; Jong, E. C.; Pottinger, P. Tuberculosis in Travelers and Immigrants. In The Travel and Tropical Medicine. 5th ed; Elsevier, 2008; pp 391–406.
- Brosch, R.; Gordon, S. V.; Marmiesse, M.; Brodin, P.; Buchrieser, C.; Eiglmeier, K.; Garnier, T.; Gutierrez, C.; Hewinson, G.; Kremer, K.; et al. A New Evolutionary Scenario for the Mycobacterium Tuberculosis Complex. Proc Natl Acad Sci U S A 2002, 99 (6), 3684–3689.
- Gutierrez, M. C.; Brisse, S.; Brosch, R.; Fabre, M.; Omaïs, B.; Marmiesse, M.; Supply, P.; Vincent, V. Ancient Origin and Gene Mosaicism of the Progenitor of Mycobacterium Tuberculosis. PLoS Pathog 2005, 1 (1), 55–61.
- Delogu, G.; Sali, M.; Fadda, G. The Biology of Mycobacterium Tuberculosis Infection. Mediterr J Hematol Infect Dis 2013, 5 (1), 2935–3006.
- Miller, P. B.; Zalwango, S.; Galiwango, R.; Kakaire, R.; Sekandi, J.; Steinbaum, L.; Drake, J. M.; Whalen, C. C.; Kiwanuka, N. Association between Tuberculosis in Men and Social Network Structure in Kampala, Uganda. BMC Infect Dis 2021, 21 (1), 1–9.
- Marçôa, R.; Ribeiro, A. I.; Zão, I.; Duarte, R. Tuberculosis and Gender – Factors Influencing the Risk of Tuberculosis among Men and Women by Age Group. Pulmonology 2018, 24 (3), 199–202.
- 2024 WHO Global Tuberculosis Report; 2024.
- Mason, E. Towards a New Class of Anti-Tuberculosis Drugs: Design and Synthesis of AnPRT TS Analogues, Te Herenga Waka-Victoria University of Wellington, 2023.
- Maitra, A.; Munshi, T.; Healy, J.; Martin, L. T.; Vollmer, W.; Keep, N. H.; Bhakta, S. Cell Wall Peptidoglycan in Mycobacterium Tuberculosis: An Achilles’ Heel for the TB-Causing Pathogen. FEMS Microbiol Rev 2019, 43 (5), 548–575.
- Cobat, A.; Gallant, C. J.; Simkin, L.; Black, G. F.; Stanley, K.; Hughes, J.; Doherty, T. M.; Hanekom, W. A.; Eley, B.; Jaïs, J. P.; et al. Two Loci Control Tuberculin Skin Test Reactivity in an Area Hyperendemic for Tuberculosis. Journal of Experimental Medicine 2009, 206 (12), 2583–2591.
- Gengenbacher, M.; Kaufmann, S. H. E. Mycobacterium Tuberculosis: Success through Dormancy. FEMS Microbiol Rev 2012, 36 (3), 514–532.
- Neyrolles, O.; Hernández-Pando, R.; Pietri-Rouxel, F.; Fornès, P.; Tailleux, L.; Payán, J. A. B.; Pivert, E.; Bordat, Y.; Aguilar, D.; Prévost, M. C.; et al. Is Adipose Tissue a Place for Mycobacterium Tuberculosis Persistence? PLoS One 2006, 1 (1), 1–9.
- Ottenhoff, T. H. M.; Kaufmann, S. H. E. Vaccines against Tuberculosis: Where Are We and Where Do We Need to Go? PLoS Pathog 2012, 8 (5), 1–12.
- Russell, D. G. Mycobacterium Tuberculosis and the Intimate Discourse of a Chronic Infection. Immunol Rev 2011, 240 (1), 252–268.
- Tiberi, S.; du Plessis, N.; Walzl, G.; Vjecha, M. J.; Rao, M.; Ntoumi, F.; Mfinanga, S.; Kapata, N.; Mwaba, P.; McHugh, T. D.; et al. Tuberculosis: Progress and Advances in Development of New Drugs, Treatment Regimens, and Host-Directed Therapies. Lancet Infect Dis 2018, 18 (7), 183–198.
- Pai, M.; Behr, M. A.; Dowdy, D.; Dheda, K.; Divangahi, M.; Boehme, C. C.; Ginsberg, A.; Swaminathan, S.; Spigelman, M.; Getahun, H.; et al. Tuberculosis. Nature Reviews Disease Primers 2016 2:1 2016, 2 (1), 1–23.
- Vynnycky, E.; Fine, P. E. M. The Natural History of Tuberculosis: The Implications of Age-Dependent Risks of Disease and the Role of Reinfection. Epidemiol Infect 1997, 119 (2), 183–201.
- Lin, P. L.; Ford, C. B.; Coleman, M. T.; Myers, A. J.; Gawande, R.; Ioerger, T.; Sacchettini, J.; Fortune, S. M.; Flynn, J. L. Sterilization of Granulomas Is Common in Active and Latent Tuberculosis despite Within-Host Variability in Bacterial Killing. Nat Med 2014, 20 (1), 75–79.
- Dowdy, D. W.; Basu, S.; Andrews, J. R. Is Passive Diagnosis Enough? The Impact of Subclinical Disease on Diagnostic Strategies for Tuberculosis. Am J Respir Crit Care Med 2013, 187 (5), 543–551.
- Palomino, J. C.; Martin, A. Drug Resistance Mechanisms in Mycobacterium Tuberculosis. Antibiotics 2014, 3 (3), 317–340.
- Gygli, S. M.; Borrell, S.; Trauner, A.; Gagneux, S. Antimicrobial Resistance in Mycobacterium Tuberculosis: Mechanistic and Evolutionary Perspectives. FEMS Microbiol Rev 2017, 41 (3), 354–373.
- Kendall, E. A.; Fofana, M. O.; Dowdy, D. W. Burden of Transmitted Multidrug Resistance in Epidemics of Tuberculosis: A Transmission Modelling Analysis. Lancet Respir Med 2015, 3 (12), 963–972.
- Daniels, M.; Hill, A. B. Chemotherapy of Pulmonary Tuberculosis in Young Adults. Br Med J 1952, 1 (4769), 1162–1168.
- Luca, S.; Mihaescu, T. History of BCG Vaccine. Maedica (Bucur) 2013, 8 (1), 53–58.
- Moliva, J. I.; Turner, J.; Torrelles, J. B. Immune Responses to Bacillus Calmette-Guérin Vaccination: Why Do They Fail to Protect against Mycobacterium Tuberculosis? Front Immunol 2017, 8 (407), 1–17.
- Dockrell, H. M.; Smith, S. G. What Have We Learnt about BCG Vaccination in the Last 20 Years? Front Immunol 2017, 8 (1134), 1–10.
- Heo, J.; Koh, D.; Woo, M.; Kwon, D.; Carla De Almeida Falcão, V.; Wood, C.; Lee, H.; Kim, K.; Choi, I.; Jang, J.; et al. A Combination Screening to Identify Enhancers of Para-Aminosalicylic Acid against Mycobacterium Tuberculosis. Sci Rep 2022, 12 (5635), 1–14.
- McCarthy, O. R. The Key to the Sanatoria. J R Soc Med 2001, 94 (8), 413–417.
- Murray, J. F.; Schraufnagel, D. E.; Hopewell, P. C. Treatment of Tuberculosis: A Historical Perspective. Ann Am Thorac Soc 2015, 12 (12), 1749–1759.
- Lee, A.; Xie, Y. L.; Barry, C. E.; Chen, R. Y. Current and Future Treatments for Tuberculosis. BMJ 2020, 368 (216), 1–18.
- World Health Organization. Global Tuberculosis Report 2018; WHO, 2018.
- Sulis, G.; Centis, R.; Sotgiu, G.; D’Ambrosio, L.; Pontali, E.; Spanevello, A.; Matteelli, A.; Zumla, A.; Migliori, G. B. Recent Developments in the Diagnosis and Management of Tuberculosis. NPJ Prim Care Respir Med 2016, 26 (1), 1–8.
- Hagga, M. A. M.; Sultana, S. A Novel Quantitative Method for the Simultaneous Assay of Rifampicin (RIF), Isoniazid (INH), Ethambutol (EMB), and Pyrazinamide (PYP) in 4-FDC Tablets. Oriental Journal of Chemistry 2016, 32 (6), 3081–3087.
- Treatment of drug-susceptible tuberculosis: rapid communication https://www.who.int/publications/i/item/9789240028678 (accessed 07/12/2021).
- Priftin (Rifapentine): Uses, Dosage, Side Effects, Interactions, Warnings https://www.rxlist.com/priftin-drug.htm#description (accessed 07/12/2021).
- Barman Balfour, J. A.; Lamb, H. M. Moxifloxacin: A Review of Its Clinical Potential in the Management of Community-Acquired Respiratory Tract Infections. Drugs 2000, 59 (1), 115–139.
- Munsiff, S. S.; Kambili, C.; Ahuja, S. D. Rifapentine for the Treatment of Pulmonary Tuberculosis. Clinical Infectious Diseases 2006, 43 (11), 1468–1475.
- Tang, P.; Johnston, J. Treatment of Latent Tuberculosis Infection. Curr Treat Options Infect Dis 2017, 9 (4), 371–379.
- Module 4: Treatment - Drug-Resistant Tuberculosis Treatment https://www.who.int/publications/i/item/9789240006997 (accessed 08/12/2021).
- Nahid, P.; Mase, S. R.; Migliori, G. B.; Sotgiu, G.; Bothamley, G. H.; Brozek, J. L.; Cattamanchi, A.; Peter Cegielski, J.; Chen, L.; Daley, C. L.; et al. Treatment of Drug-Resistant Tuberculosis an Official ATS/CDC/ERS/IDSA Clinical Practice Guideline. Am J Respir Crit Care Med 2019, 200 (10), 93–142.
- Migliori, G. B.; Langendam, M. W.; D’Ambrosio, L.; Centis, R.; Blasi, F.; Huitric, E.; Manissero, D.; van der Werf, M. J. Protecting the Tuberculosis Drug Pipeline: Stating the Case for the Rational Use of Fluoroquinolones. European Respiratory Journal 2012, 40 (4), 814–822.
- Shen, L. L. Molecular Mechanisms of DNA Gyrase Inhibition by Quinolone Antibacterials. Adv Pharmacol 1994, 29, 285–304.
- Hashemian, S. M. R.; Farhadi, T.; Ganjparvar, M. Linezolid: A Review of Its Properties, Function, and Use in Critical Care. Drug Des Devel Ther 2018, 12, 1759–1767.
- Koul, A.; Dendouga, N.; Vergauwen, K.; Molenberghs, B.; Vranckx, L.; Willebrords, R.; Ristic, Z.; Lill, H.; Dorange, I.; Guillemont, J.; et al. Diarylquinolines Target Subunit c of Mycobacterial ATP Synthase. Nature Chemical Biology 2007 3:6 2007, 3 (6), 323–324.
- Guglielmetti, L.; Jaspard, M.; le Dû, D.; Lachâtre, M.; Marigot-Outtandy, D.; Bernard, C.; Veziris, N.; Robert, J.; Yazdanpanah, Y.; Caumes, E.; et al. Long-Term Outcome and Safety of Prolonged Bedaquiline Treatment for Multidrug-Resistant Tuberculosis. European Respiratory Journal 2017, 49 (3), 1–11.
- Clofazimine: Uses, Interactions, Mechanism of Action https://go.drugbank.com/drugs/DB00845 (accessed Dec 8, 2021).
- Nitsche, M. A.; Jaussi, W.; Liebetanz, D.; Lang, N.; Tergau, F.; Paulus, W. Consolidation of Human Motor Cortical Neuroplasticity by D-Cycloserine. Neuropsychopharmacology 2004 29:8 2004, 29 (8), 1573–1578.
- Prosser, G. A.; de Carvalho, L. P. S. Kinetic Mechanism and Inhibition of Mycobacterium Tuberculosis D-Alanine:D-Alanine Ligase by the Antibiotic d-Cycloserine. FEBS J 2013, 280 (4), 1150–1166.
- Kitamura, Y.; Ebihara, A.; Agari, Y.; Shinkai, A.; Hirotsu, K.; Kuramitsu, S. Structure of D-Alanine-d-Alanine Ligase from Thermus Thermophilus HB8: Cumulative Conformational Change and Enzyme–Ligand Interactions. Acta Crystallogr D Biol Crystallogr 2009, 65, 1098–1106.
- Ramanathan, M. R.; Howell, C. K.; Sanders, J. M. Drugs in Tuberculosis and Leprosy. Side Effects of Drugs Annual 2019, 41, 321–338.
- Delamanid - an overview https://www.sciencedirect.com/topics/medicine-and-dentistry/delamanid (accessed 09/12/2021).
- 28. Drugs Used in Tuberculosis and Leprosy. In Side Effects of Drugs Annual 38; Ray, S. D., Ed.; ELSEVEIR, 2016; p 284.
- Imipenem and Cilastatin Injection https://medlineplus.gov/druginfo/meds/a686013.html (accessed 04/01/2022).
- Meropenem (Merrem) - Side Effects, Interactions, Uses, Dosage, Warnings https://www.everydayhealth.com/drugs/meropenem (accessed 04/01/2022).
- Serio, A. W.; Keepers, T.; Andrews, L.; Krause, K. M. Aminoglycoside Revival: Review of a Historically Important Class of Antimicrobials Undergoing Rejuvenation. EcoSal Plus 2018, 8 (1), 1–20.
- Sabur, N. F.; Brar, M. S.; Wu, L.; Brode, S. K. Low-Dose Amikacin in the Treatment of Multidrug-Resistant Tuberculosis (MDR-TB). BMC Infect Dis 2021, 21 (1), 1–8.
- Peloquin, C. A. Medication Fact Sheets. Drug-Resistant Tuberculosis: A Survival Guide for Clinicians 2016, 99–148.
- Cohen, K. A.; Stott, K. E.; Munsamy, V.; Manson, A. L.; Earl, A. M.; Pym, A. S. Evidence for Expanding the Role of Streptomycin in the Management of Drug-Resistant Mycobacterium Tuberculosis. Antimicrob Agents Chemother 2020, 64 (9), 1–10.
- Ethionamide (Eto) and Prothionamide (Pto) - Tuberculosis https://medicalguidelines.msf.org/viewport/TUB/latest/ethionamide-eto-and-prothionamide-pto-20324029.html (accessed 08/01/2022).
- Li, G.; Zhang, J.; Jiang, Y.; Zhao, L. li; Liu, H.; Li, M.; Zhao, X.; Wan, K. Cross-Resistance of Isoniazid, Para-Aminosalicylic Acid and Pasiniazid against Isoniazid-Resistant Mycobacterium Tuberculosis Isolates in China. J Glob Antimicrob Resist 2020, 20, 275–281.
- Luo, M.; Li, K.; Zhang, H.; Yan, X.; Gu, J.; Zhang, Z.; Chen, Y.; Li, J.; Wang, J.; Chen, Y. Molecular Characterization of Para-Aminosalicylic Acid Resistant Mycobacterium Tuberculosis Clinical Isolates in Southwestern China. Infect Drug Resist 2019, 12, 2269–2275.
- Donald, P. R.; Diacon, A. H. Para-Aminosalicylic Acid: The Return of an Old Friend. Lancet Infect Dis 2015, 15 (9), 1091–1099.
- Para-aminosalicylic acid (PAS) and sodium salt of PAS - Tuberculosis https://medicalguidelines.msf.org/viewport/TUB/latest/para-aminosalicylic-acid-pas-and-sodium-salt-of-pas-20324051.html (accessed 08/01/2022).
- WHO announces updated definitions of extensively drug-resistant tuberculosis https://www.who.int/news/item/27-01-2021-who-announces-updated-definitions-of-extensively-drug-resistant-tuberculosis (accessed 09/01/2022).
- Treatment of extensively drug-resistant TB (XDR-TB) - Tuberculosis https://medicalguidelines.msf.org/viewport/TUB/latest/10-10-treatment-of-extensively-drug-resistant-tb-xdr-tb-20322528.html (accessed 09/01/2022).
- NHS England. Clinical Commissioning Policy Statement: Treatment for Defined Patients with MDR-TB and XDR-TB Including Bedaquiline and Delamanid NHS England Reference: 201203P 2 Clinical Commissioning Policy Statement: Treatment for Defined Patients with MDR-TB and XDR-TB Including Bedaquiline and Delamanid Prepared by NHS England Specialised Services Clinical Reference Group for Infectious Diseases; 2019.
- What is the cure rate for multidrug-resistant tuberculosis (MDR-TB)? https://www.medscape.com/answers/230802-19469/what-is-the-cure-rate-for-multidrug-resistant-tuberculosis-mdr-tb (accessed 09/01/2022).
- World Health Organization. Global Tuberculosis Report 2021; WHO, 2021.