Abstract
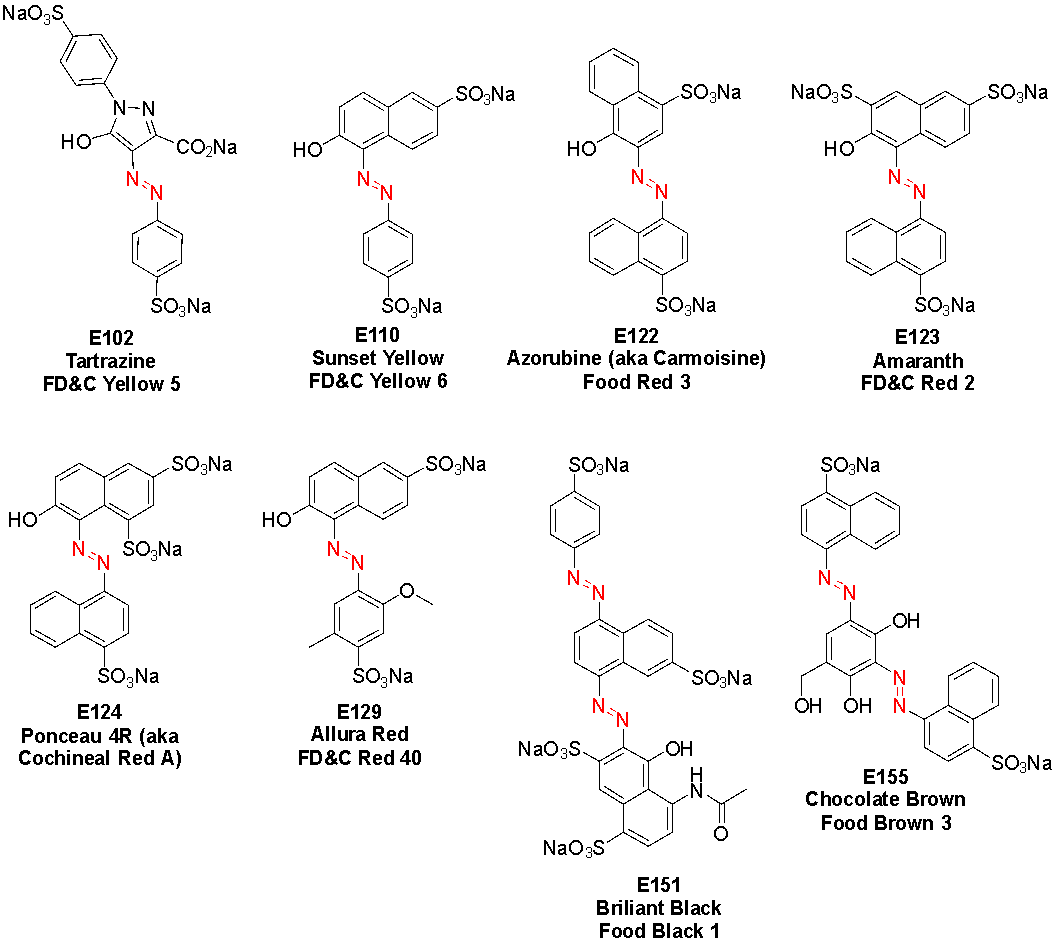
Azo dyes are vibrant synthetic colourants that enjoy widespread application in processed food and drinks, textiles, cosmetics and pharmaceuticals. In New Zealand, eight azo dyes are permitted for inclusion in food and drinks: tartrazine (E102), sunset yellow (E110), azorubine (E122), amaranth (E123), ponceau 4R (E124), allura red (E129), brilliant black (E151) and chocolate brown (E155). These are sulfonated, conjugated aromatic molecules containing an azo (N=N) bond, which is susceptible to reduction by microbial and abiotic reductants in the gut. The toxicology of both the original dye and the reduction metabolites must be considered in understanding the health effects of these food additives. While concerns about long-term or cancerous effects are not confirmed, there are definite links with neurobehavioural and immunological effects in some consumers.
Introduction
The USA Health Secretary, R. F. Kennedy, has recently announced a ban on eight artifical food dyes (https://www.bbc.com/news/articles/cpdzyjyp8x1o).1 In practice, this ban will be implemented by revoking approval by the US Food and Drug Administration (FDA) and/or removal of the colourants from the food supply chain. This follows announcements by the states of California and West Virginia that food and drinks containing certaining synthetic dyes will be removed from school cafeteria and snack machines amidst health concerns (Newsom signs bill to expel six food dyes from California schools - Los Angeles Times; US's unhealthiest state passes ban on cancer-linked food ingredients).2,3 Ongoing consumer alarm about the health effects of synthetic colourants in processed foods and beverages, especially on children who are attracted to the bright colours, is felt globally, including in New Zealand (https://www.stuff.co.nz/life-style/well-good/4169217/Seeing-red-over-additives).4 Consumption of synthetic dyes is linked with health impacts such as ADHD, asthma and other immune conditions.5–9 Legislative or consumer-driven change has been driven by scientific studies reporting these links. For example, a 2007 study conducted in Southampton in the UK showed increased hyperactivity in children upon consumption of processed food containing synthetic dyes and the common preservative sodium benzoate.10 This led the British Food Standards Agency in 2008 to recommend the removal of the synthetic colourants included in the Southampton study (known as the “Southampton 6”) (https://www.theguardian.com/uk/2008/apr/10/foodanddrink).11 Although these dyes are still legally permitted as food colourants,12 the UK food industry complied with the recommendation by largely phasing out the dyes.
Similarly, a 2022 meta-study from the California Environmental Protection Agency that showed a correlation between synthetic food dyes and adverse behavioural changes in children5 has likely played a part in the current regulatory activity in the USA. Removal or replacement of synthetic colourants in processed foods is a complex matter under active investigation by manufacturers (Snack Makers Are Removing Fake Colors From Processed Foods),13 yet the changes in Britain11 demonstrate that change is possible.
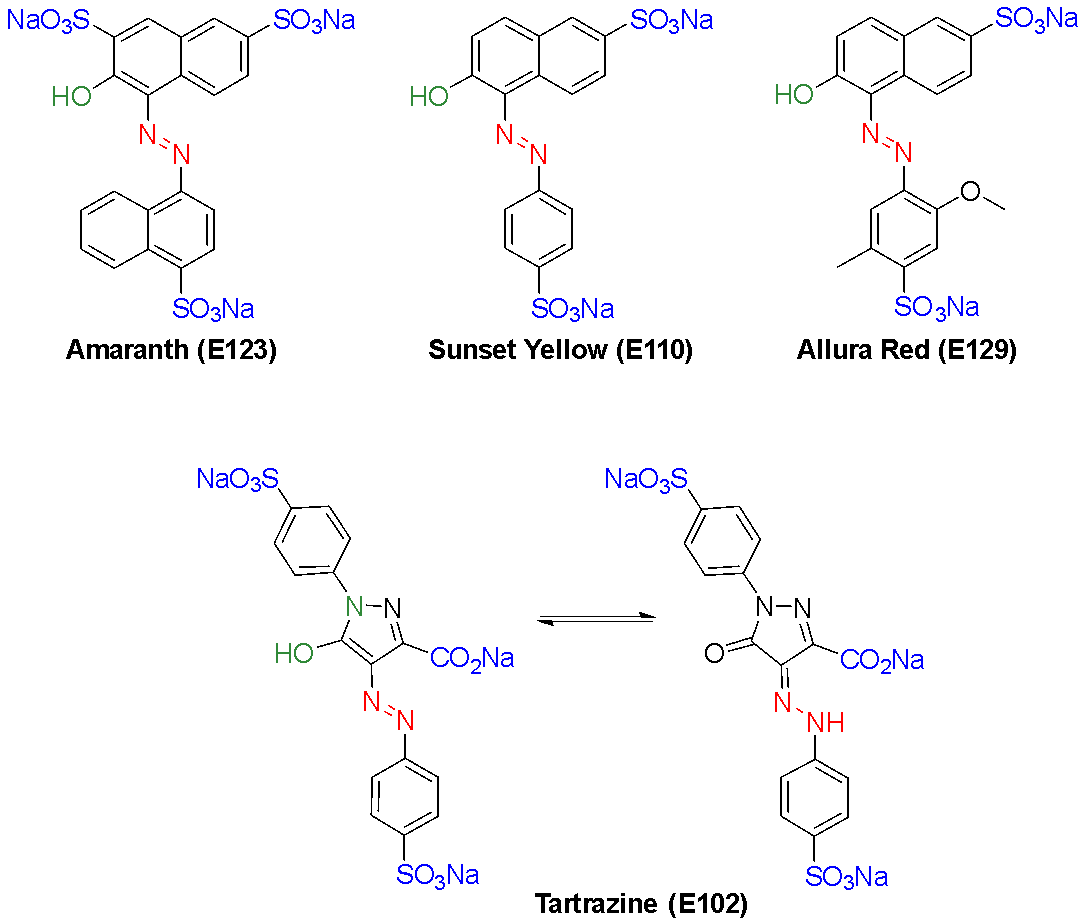
The synthetic dyes under the spotlight are conjugated aromatic compounds bearing polar sulfonate and/or carboxylate groups, which enhance aqueous solubility as needed for ingestion. Four of the dyes announced for removal in the USA contain an azo functional group and are known as azo dyes: tartrazine (E102), sunset yellow (E110), amaranth (E123) and allura red (E129). These are the only azo dyes permitted in food and drink in the USA: the FDA has phased out, or never approved, other azo food dyes. In fact, the promise to revoke authorisation for amaranth within weeks1 is arguably a political stunt, since it was delisted by the FDA in 1976 and since then should only have been included in food destined for export from the USA!14 In Canada and Europe, all four of these dyes are permitted. The New Zealand and Australian food standards code permits the inclusion of a total of eight azo dyes in food and drink (Fig. 1).15,16
In the midst of the announcements of changes to legislation surrounding artificial dyes,1 the science of these chemicals is worth considering. This article will examine azo dyes, focusing on their chemistry, properties, metabolism, history, and considering the New Zealand and Australian situation with regard to their use in food.
Azo compounds – properties, applications and history
Compounds containing an azo functional group are known as azo compounds and have a range of fascinating and useful properties. As featured recently in this journal, azo compounds are potentially applicable as photoswitches,17 while azo polymers are under investigation for energy storage applications.18 They are also found as active pharmaceutical ingredients in some medicines.19,20 Nonetheless, the overwhelmingly most significant role played by azo compounds is as dyes.
Azo dyes have spectacularly vibrant hues that are used extensively in the textile, food, pharmaceutical and cosmetics industries.21-25 The colour results from an electronic transition that absorbs electromagnetic radiation in the visible region (400–800 nm) of the spectrum corresponding to the wavelength/energy of the complementary colour.22 The azo bond is the chromophore, while the aromatic rings and pendant substituents are auxochromes that modulate the energy that is absorbed, providing tunability in colour.22,26
Azo compounds were discovered in the 1860s and developed as textile colourants through the 1870s.27 These synthetic dyes became popular textile colours because they were longer-lasting than the existing natural dyes. The tunable relationship between colour and the chemical substituents on the azo group has made these textile dyes very popular. Azo dyes remain vitally important for the global textile industry, making up 60–70% of dye production due to their favourable properties, especially their robust adherance to fabrics.28 They are likewise prevalent as colourants in processed food, drinks and cosmetics.20
As a result of the diverse industries that use azo dyes, they have many designated names or codes (Table 1, refer to structures in Fig. 1).29 For example, the European Union applies E numbers to food colourants (synthetic and natural) and this is the coding that New Zealand and Australia follow. The USA, however, describes food dyes with an FD&C code. In contrast, the textile industry uses a Colour Index (C.I.) number to describe dyes according to their chemical structures. Other terminology is also used in various contexts, making identification of dyes on product packaging a challenge for consumers.
Azo compounds – chemistry
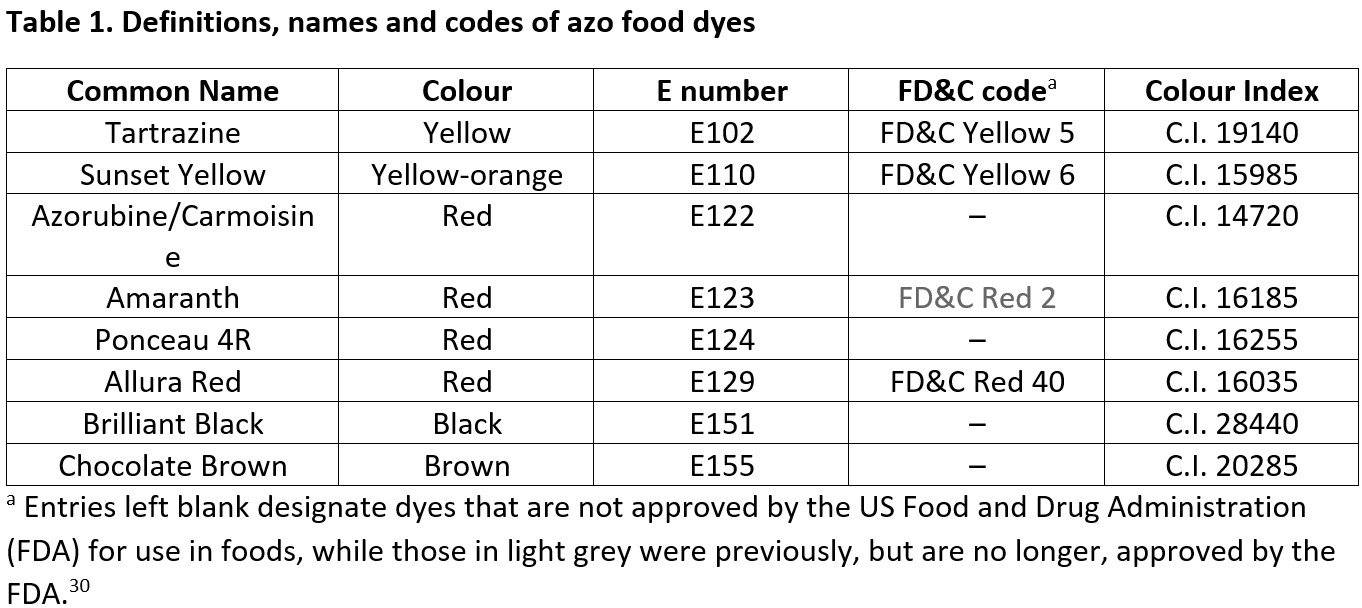
Azo compounds are prepared by reaction of a diazonium salt with an aromatic compound (Fig. 2). Mechanistically, this is driven by the reactivity of the positively charged diazonium salt – an electrophile – and constitutes an electrophilic aromatic substitution with respect to the other partner. The diazonium salt is prepared by diazotisation of an aromatic amine, typically using sodium nitrite in acid.21
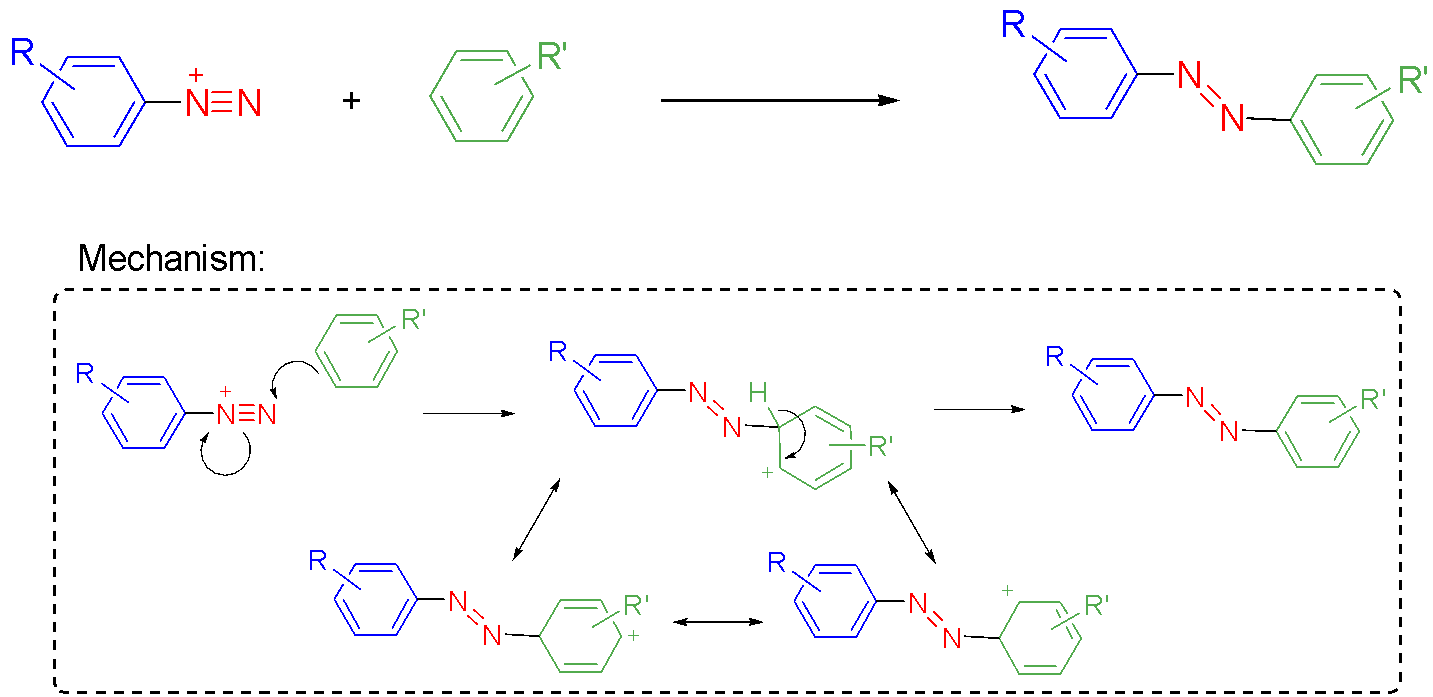
As an example, sunset yellow is manufactured by diazotising 4-aminobenzenesulfonic acid and then coupling of the resulting diazonium salt with 6-hydroxy-2-naphthalenesulfonic acid (Fig. 3).31 The dye is isolated as its disodium salt by basification with sodium hydroxide. The regioselectivity (positional isomer selectivity) in the coupling step results from the most electron-rich site on the naphthalene reacting with the diazonium salt. This electrophilic aromatic substitution of the naphthlene partner proceeds via a resonance-stabilised cationic intermediate (Fig. 3 inset). Examining the canonical structures of the intermediate reveals that coupling at this site on the naphthalene maximises stabilisation by the electron-donating phenolic OH group and minimises destabilisation by the electron-withdrawing sulfonate.
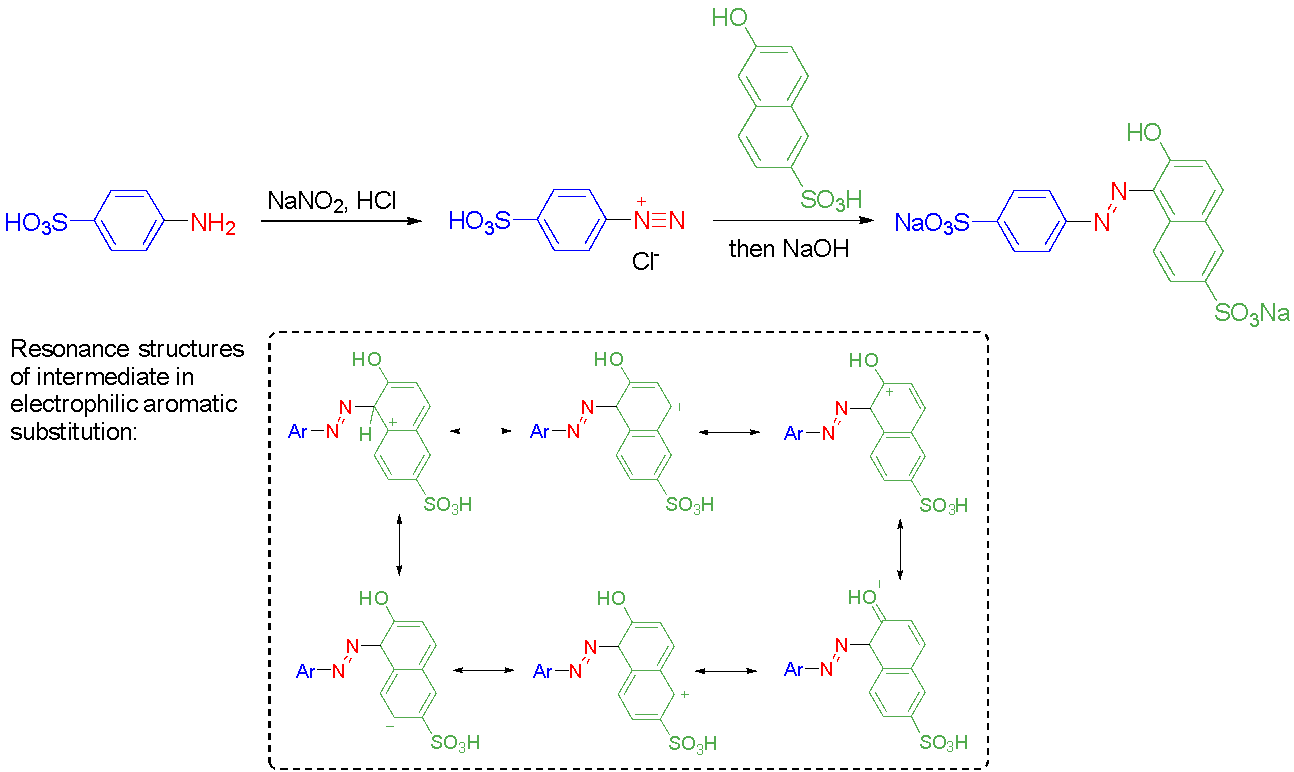
Azo compounds undergo trans-cis isomerism, often initiated by irradiation with a particular wavelength of light (Figure 4).17 The reverse isomeric process is typically spontaneous due to the energetic favouring of the sterically minimised trans-isomer, although it can be accelerated thermally.
Azo compounds are susceptible to reduction. Reductive cleavage of the azo bond results in formation of aromatic amines corresponding to the two parts of the reactive molecule (Fig. 5). This process occurs with chemical or biological reductants.32,33 Typical chemical reductants include stannous chloride, zinc powder, magnesium-palladium couple and sodium dithionite.32,34–36 Azoreductases are oxidoreductase enzymes that reduce azo compounds in addition to other substrates and are ubiquitous in bacteria.37,38 Azoreductases use NADH or NADPH as the reductant and some also require flavins as coenzymes.37,39
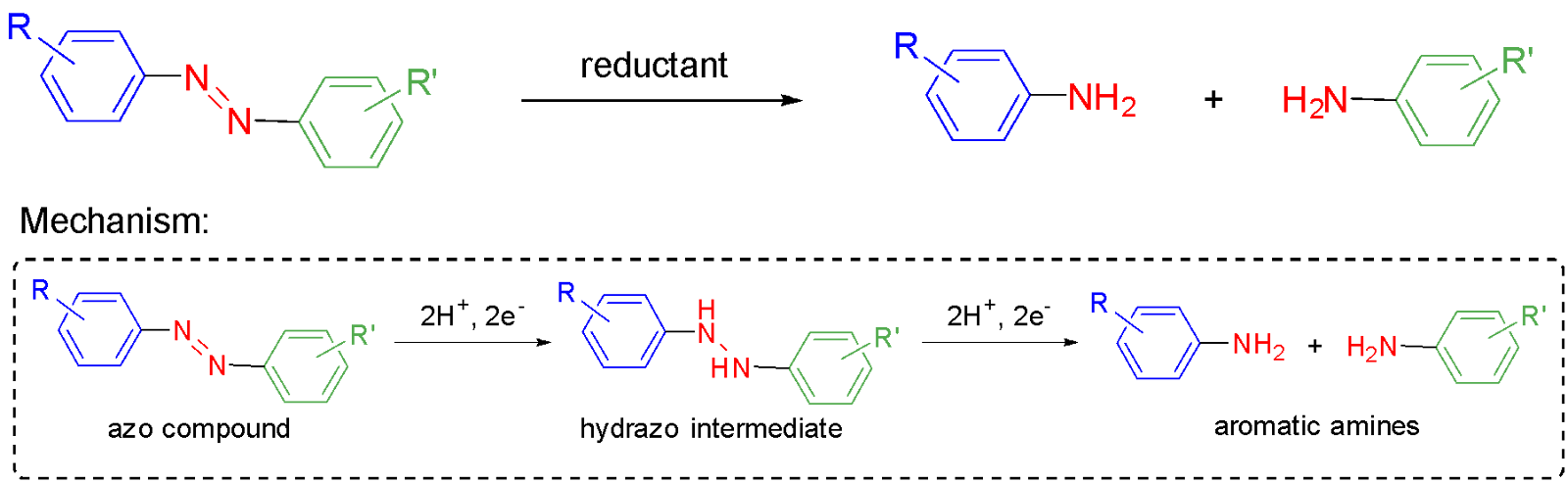
The conversion of azo compounds into amines is believed to proceed mechanistically through a stepwise four-electron reduction, via a hydrazo intermediate (Fig. 5 inset).39,40 The reduction potential of the azo bond depends on the substituents attached to the aromatic rings and the pH.40,41 Reduction is more facile for azo compounds with electron-withdrawing groups in positions that make the azo bond more electrophilic.33,40 However, some azo compounds do not reduce all the way to the amine but stall at the hydrazo intermediate. A recent study indicates that cleavage of the hydrazo N-N bond relies on resonance stabilisation by a suitably positioned electron-donating substituent through a quinone imine intermediate, which is then reduced by the reductant.42 As all azo food dyes contain at least one electron-donating hydroxyl (-OH) substituent ortho-to the azo bond (refer back to Fig. 1 structures), their complete reduction to aromatic amine end-points can be assumed.
Consideration of azo dye reduction is pertinent to treatment of wastewater streams from dye production and textile manufacture. Due to the toxicity of aromatic amines, decolourisation of waste streams by reduction is at best merely cosmetic and, in reality, is highly detrimental to downstream inhibitants, flora and fauna.20 Releasing the untreated dyes themselves not only pollutes waterways, but downstream bacterial reduction can occur,43 resulting in the same aromatic amines. Therefore, sophisticated treatment regimes are needed to decolourise and detoxify industrial wastestreams involving dyes.26,33,40,44,45
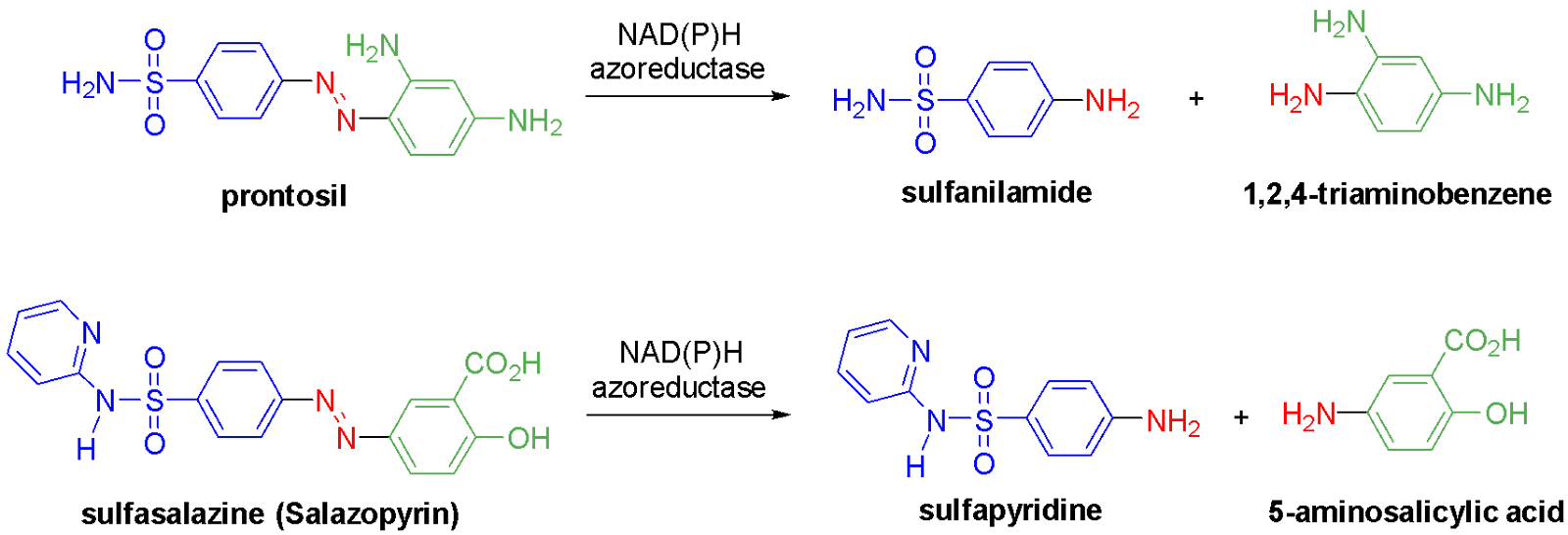
The human gut microbiome contains a multitude of azoreductases - enzymes that reduce azo compounds.46–48 Reduction of azo compounds by intestinal bacteria has been exploited for drug delivery due to the prevalence of azoreductase-containing bacteria in the human gut. This enables an azo compound prodrug to be taken orally (usually as a tablet), transported through the digestive tract and reductively metabolised into the active amine-containing medicine in the intestine. Prontosil is an antibiotic prodrug, which is metabolised into the active drug sulfanilamide in the liver and/or the colon (Fig. 6).9.20 Sulfasalazine (sold in NZ as Salazopyrin) is a treatment for rheumatoid arthritis, Crohn’s disease and ulcerative colitis. This azo compound also undergoes metabolic reduction in the colon to produce sulfapyridine and 5-aminosalicylic acid (a.k.a. mesalazine). While the individual therapeutic roles of this drug and its metabolites are not unambiguously determined, it is likely that the known anti-inflammatory 5-aminosalicylic acid delivers the desired effects against these autoimmune diseases. Administering hydrophilic 5-aminosalicylic acid as its more lipophilic prodrug minimises its absorption in the digestive tract too early and optimises its delivery to the site of action - the colon.
Reduction chemistry of azo food dyes
Azo dyes in food are reduced in the human gut by bacterial and abiotic reductants.39,42,46–48 A vast array of bacterial strains reduce the azo food dyes found in many processed foods and beverages. Variability in the rates of reduction of azo food dyes by different gut bacteria was detected spectrophotometrically.48 Furthermore, certain dyes were reduced more readily than others in a study by our collaborators with a wide panel of bacterial species, as observed by decolourisation of the dye on plates.46 These findings can be rationalised by consideration of their structures. The electron-deficient amaranth (E123), containing three sulfonate groups, was more readily reduced (by more bacterial strains) than the less electron-deficient sunset yellow (E110) and allura red (E129), which contain just two sulfonate moieties (Fig. 7).46 This fits the expectation that suitably positioned electron-withdrawing groups activate the azo bond towards reduction. All three of these dyes also contain an electron-donating hydroxyl (-OH) group at the ortho-position of the naphthalene ring relative to the azo bond, which will enable formation of a quinone imine intermediate and thus facilitate complete reduction. Allura red, the less reductively active of these, also has an electron-donating methoxy (-OCH3) substituent at the ortho-position of the benzene ring, which may deactivate the azo bond in the first reduction step. Tartrazine (E102), containing two sulfonate groups and a carboxylate, was the least readily reduced of the azo food dyes studied, although it was still decolourised by many strains. Tartrazine has an unusual structure, with a hydroxypyrazole ring attached to the azo bond that has a pyrazolone tautomeric structure (Fig. 7). Plausibly, the pyrazole ring itself is electron-donating and this leads to the diminished reductive activity relative to the other dyes tested. Regardless, across the usually diverse gut microbiome, there are numerous bacterial species that are capable of reducing all the azo food dyes. Hydrogen sulfide, produced in the gut from cysteine-rich foods such as meat, has also been shown to be reductively active towards the azo food dye allura red (E129), which reveals the prospect that abiotic reduction also occurs in the human gut.47 Therefore, while the azo dyes that are approved for use in food are considered to be safe, the toxicology of their reduction metabolites must also be determined.
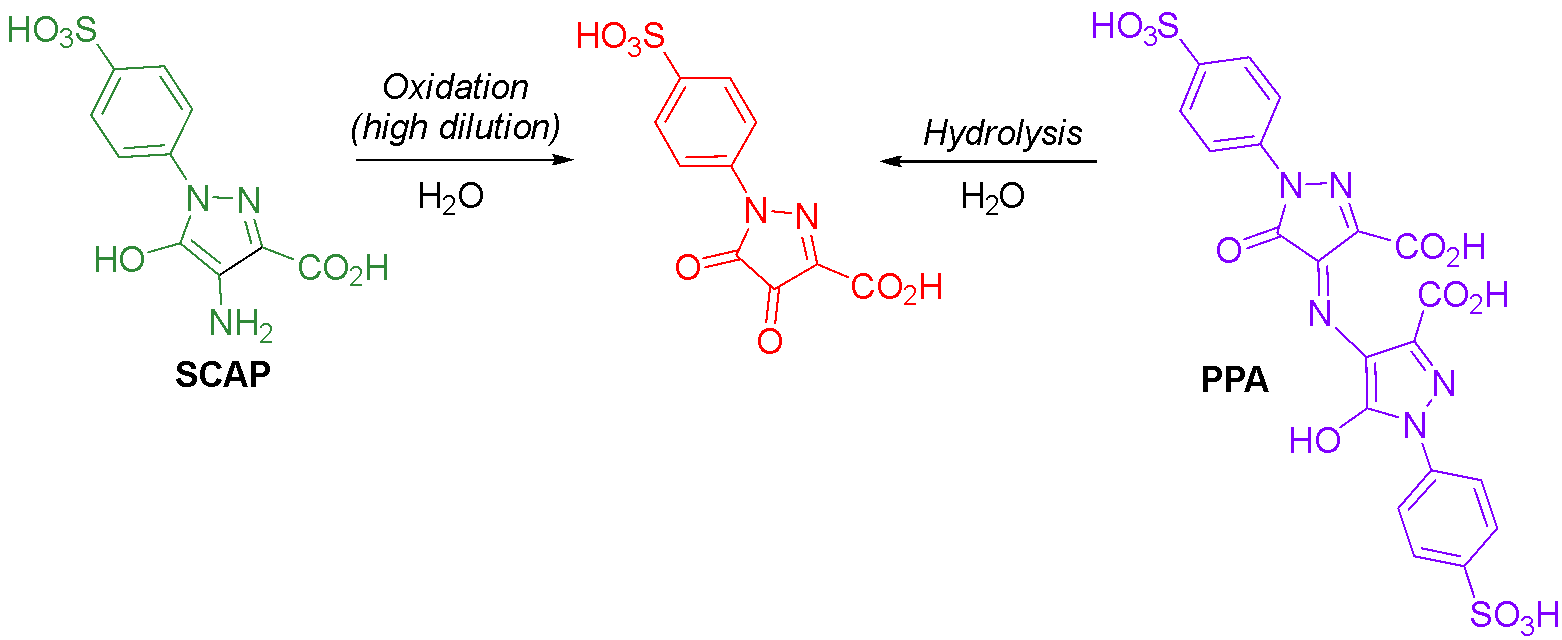
Tartrazine (E102) is the most ubiquitous of the azo food dyes, being used to produce yellow, orange and green colours in a wide variety of processed food and drinks. Bacterial reduction of tartrazine in mammalian guts produces sulfanilic acid and its further metabolite, N-acetylsulfanilic acid, which are excreted in the urine in almost quantitative combined amounts (Fig. 8).49,50 The amine metabolite derived from the pyrazolone moiety, SCAP (1-p-sulfophenyl-3-carbox-4-amino-5-pyrazolone, a.k.a. 4-amino-3-carboxy-5-hydroxy-1-(4-sulfophenyl)pyrazole), is colourless.35,51 However, once exposed to air, it produces an intensely purple-coloured species that develops visually over time in the excreted faeces.35,51 A 1965 study of tartrazine reduction identified SCAP, proposed the structure of the purple-coloured oxidation product as the dimer of SCAP less one molar equivalent of ammonia, and measured the release of ammonia.35 Our team has recently confirmed these findings through isolation and characterisation of SCAP in anaerobic conditions, followed by oxidation of SCAP to afford purple PPA (purpurazoic acid, a name coined by collaborator David Josephy).51 The formation of PPA requires a molecule of SCAP to react with a molecule of its oxidised form through an imine (Schiff’s base) transposition, resulting in the loss of an equivalent of ammonia; we could observe this process occurring with NMR spectroscopy.51
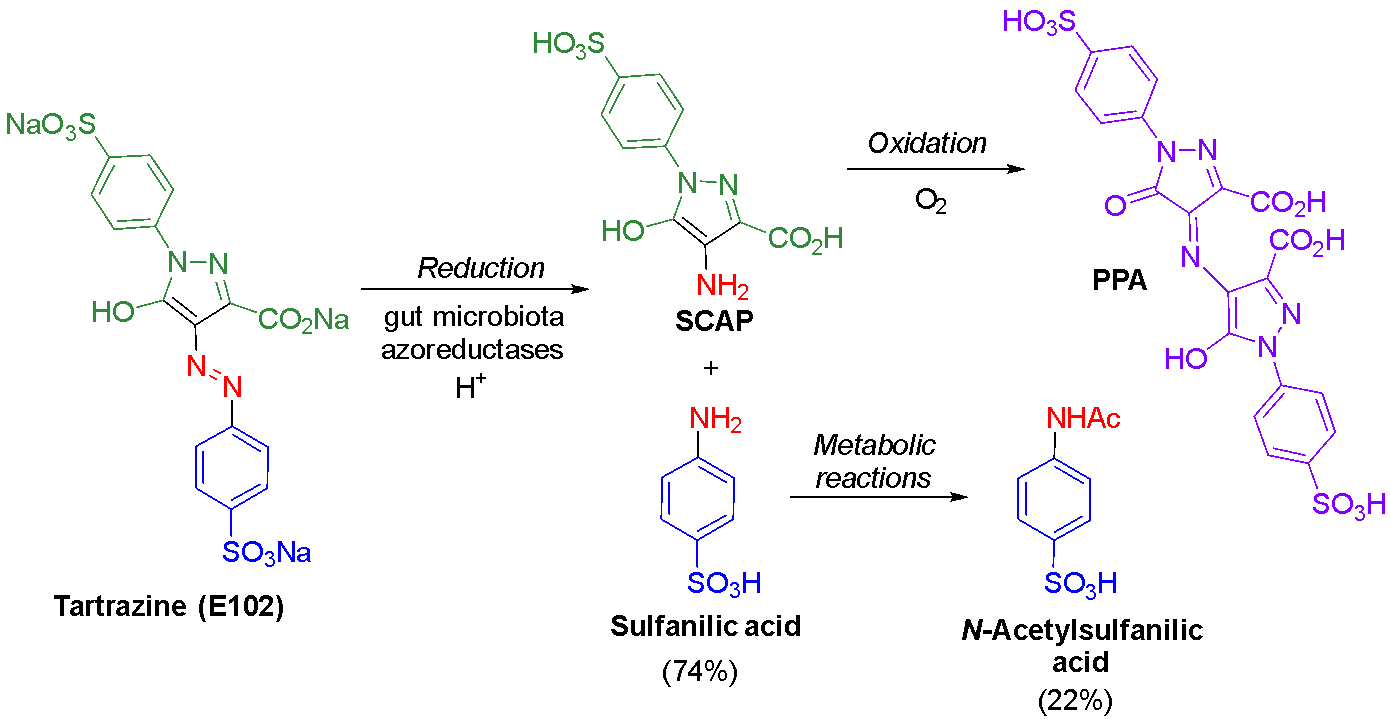
Reduction of tartrazine mediated by lactobacilli bacteria has been responsible for red colouration developing in contaminated batches of pickles.52,53 Pickle brine is typically coloured pale yellow with either tartrazine or turmeric, and the red spoilage only occurred with the former colourant.52 When this process was modelled in a brine solution coloured with tartrazine and treated with lactobacilli under anaerobic conditions, the tartrazine solution decolourised.53 Upon exposure to air, the solution became purple coloured, and the reported UV-visible and mass spectra correspond to those we obtained for PPA51 and are consistent with its structure (although the structure was not determined in the pickles study). We have observed that aqueous solutions of purple PPA degrade over time to a red colour and that SCAP forms a yellow species if oxidised at high dilution.51 Our data obtained from the product of the latter process indicate the ketone form of oxidised SCAP, which could result by hydrolysis of the initially generated imine from oxidation of SCAP or by hydrolysis of PPA (Fig. 9).
Health effects of azo food dyes and reduction metabolites
Azo food dyes tend to contain polar groups, particularly sulfonates and carboxylates, enhancing their aqueous solubilities as needed for incorporation in food and drink to pass through the digestive system. This polarity is expected to minimise their absorption systemically, including their transport to the liver,54 by disfavouring passage through hydrophobic membranes. Thus, azo food dyes are considered unlikely to have significant adverse effects on humans at doses below the acceptable daily intake (ADI).54–56 Nonetheless, problematic connections between human health and azo dyes have been seen.9
Historical safety concerns with azo dyes included observations of high incidence of bladder cancers in factory workers exposed to aromatic amines used to manufacture the dyes.20,57 Furthermore, the incidence of intestinal cancers was correlated to societies consuming more processed foods, with a possible link to colour components and azo dyes.48 However, no substantive correlations between azo dyes approved for food use and cancer have been found.56
Allergies,7,58,59 inflammation of the digestive system,6 other immune reactions,8 liver toxicity60–62 and memory deficiency63 have all been linked to azo food dyes. Strong relationships exist between the intake of certain food dyes and hyperactivity, attention deficit and other neurobehavioural issues, especially in children.5,10
Azo food colourants are metabolised in mammalian guts to aromatic amines,34,48 a class of compounds that includes toxic and carcinogenic substances.57,64 As both bacterial and abiotic components of the human gut are reductively competent,46–48 the toxicology of azo food dyes must also consider the effects of these metabolites. The extent of reduction reported varies across studies, from complete reduction49,50 to only partial conversion,56 highlighting the complexity of determining the relative impacts of the various components.23
Sulfanilic acid (structure in Fig. 8), one of the reduction products of both tartrazine and sunset yellow, shows adverse effects on human cells in vitro, causing impairment of biochemical processes and oxidative stress, albeit at rather high concentrations.65 Yet, being a sulfonated aromatic amine and therefore polar, sulfanilic acid is expected to have limited absorption in vivo beyond the digestive tract.55 It is not genotoxic nor does it cause acute toxicity.56 Sulfanilic acid, along with azo food dyes containing this substructure, did inhibit cholinesterase enzymes that are involved in neurotransmission, which suggests a link with the hyperactivity and other neurobehavioural changes seen in some consumers of artificial colourants.66
The other reduction product of tartrazine is the pyrazole amine SCAP (structure in Fig. 9). The sensitivity of SCAP to air oxidation has made it challenging to study without interference from the purple oxidation derivative PPA. We now have stocks of SCAP, kept under argon, in my lab. Collaborators in New Zealand (Professor David Ackerley, VUW) and Canada (Professor Emma Allen-Vercoe, Guelph) and their research groups are studying the effects of SCAP on human cells and gut bacteria. As we reported recently,51 SCAP and PPA are moderately toxic to human (HEK-293) cells in vitro, with a 50% inhibition of metabolic activity occurring at 89 and 78 µmolL-1 concentration. With this information, we determined that, based on US data, a child consuming several servings per day of processed foods and artificially coloured drinks67,68 could generate SCAP in their gut to toxic levels, altering the metabolism of human cells.51
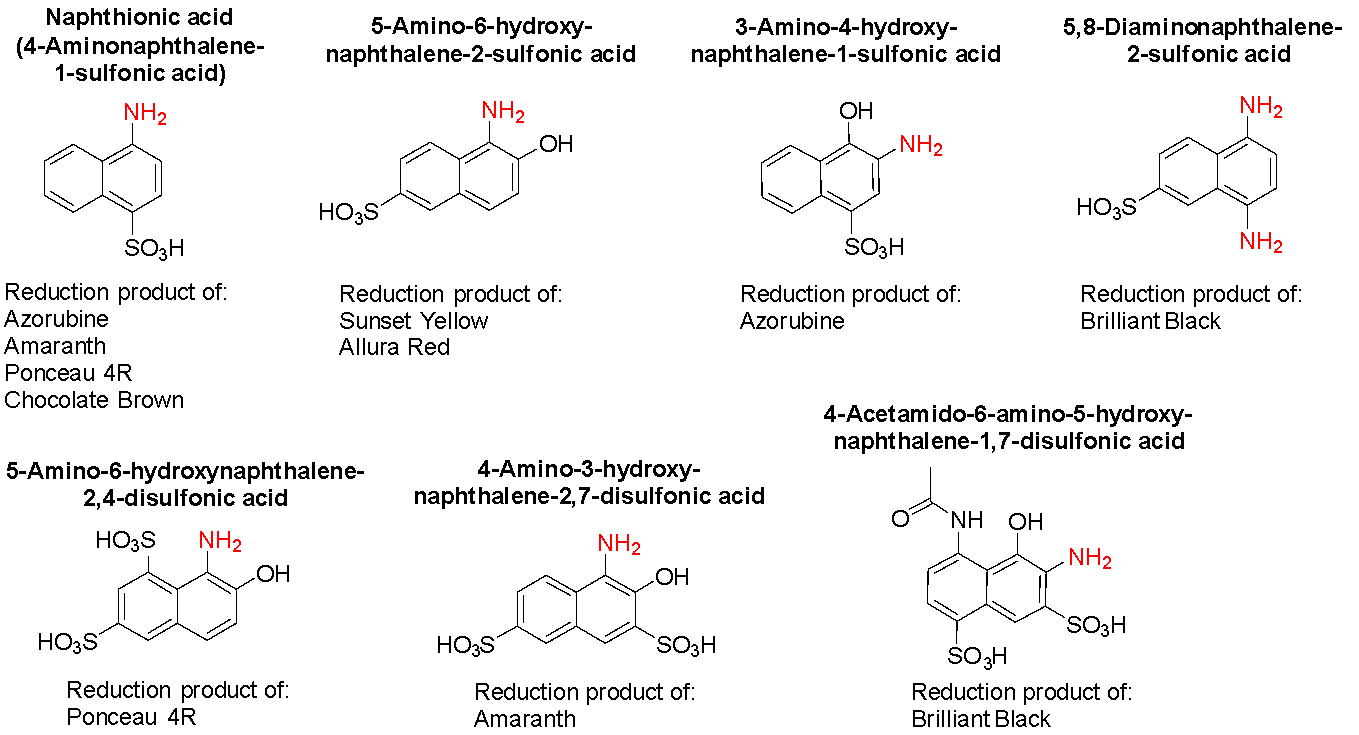
The other group of reduction metabolites from azo food dyes comprises naphthionic acid plus isomers and derivatives thereof (Fig. 10). The toxicities of these compounds are not well established, although the Australian Department of Health proposed that, for amaranth, data available for the parent dye might be used as a surrogate of the reduction metabolites.54 By way of a summary, rats fed high doses of amaranth displayed a number of changes in metabolism and activity, including a reduction in weight, likely due to decreased utilisation or absorption of nutrients, reduction in urine output, and inflammation of various organs.54 Naphthionic acid and a parent dye, azorubine, inhibited cholinesterase enzymes with a potential link to neurobehavioural changes.66
The diverse microbial environment of the gut is crucial to human health.69,70 Sulfanilamide, an aromatic amine generated from the azo compound prontosil by reduction in the gut, is an antibiotic.9,20 Given the structural and functional similarity to sulfanilamide of the amines that would derive from reduction of azo food dyes, a diet containing azo food dyes could plausibly have adverse effects on the gut microbiome. Indeed, distinct changes in the gut microbiome of carp were seen when tartrazine was consumed: loss of beneficial bacteria and increased pathogenic bacterial species occurred, together with increased inflammation markers.71 In the human gut, recent work by collaborators in Canada demonstrated that growth of some bacterial strains is decreased by azo food dyes tartrazine and sunset yellow.46 Thus, either the dyes themselves or a reduction metabolite are detrimental to these bacteria. Both of these azo dyes produce sulfanilic acid upon complete reduction, which may be a source of the gut microbiome effects.72
Overall, the complexity of studying azo dyes and their metabolites in physiological systems has led to varied outcomes from different studies. As a result, there are conflicting views on the health impacts of azo food dyes. While the breadth of evidence indicates that azo dyes authorised for use in food are not carcinogenic or mutagenic, distinct changes to metabolism and activity and increased inflammatory conditions are linked to high intake of the dyes, and gut bacteria seem to be adversely affected. Some individuals are affected more than others, and it is plausible that this variability is due to differences in gut microbiota. Given that artificial colourants produce no nutritional or flavour benefit, and may cause harm, it seems reasonable to minimise processed food and drinks containing azo dyes in our diets.
Azo dyes in NZ and Australian food and beverages
So, given the potential health impacts of azo dyes in food and drink, what is the local situation? How much are New Zealanders exposed to and affected by azo food dyes?
New Zealand and Australia have a shared Food Standards Code, adminstered through Food Standards Australia New Zealand (Te Mana Kounga Kai – Ahitereiria me Aotearoa, FSANZ). This specifies the additives that are permitted in food here. The code allows the eight azo dyes with structures shown in Fig. 1 to be present in food and drink. It requires that added colourants must be listed on food labels and it specifies maximum permitted level (MPL) in foodstuffs (as milligrams per kilogram) and drinks (usually as milligrams per litre) as listed in Table 2. Our standards refer to the United Nations Joint Expert Committee on Food Additives (JECFA)73 for acceptable daily intake (ADI) values, which are measured as milligrams per kilogram of body weight (mg/ kg bw) per day.
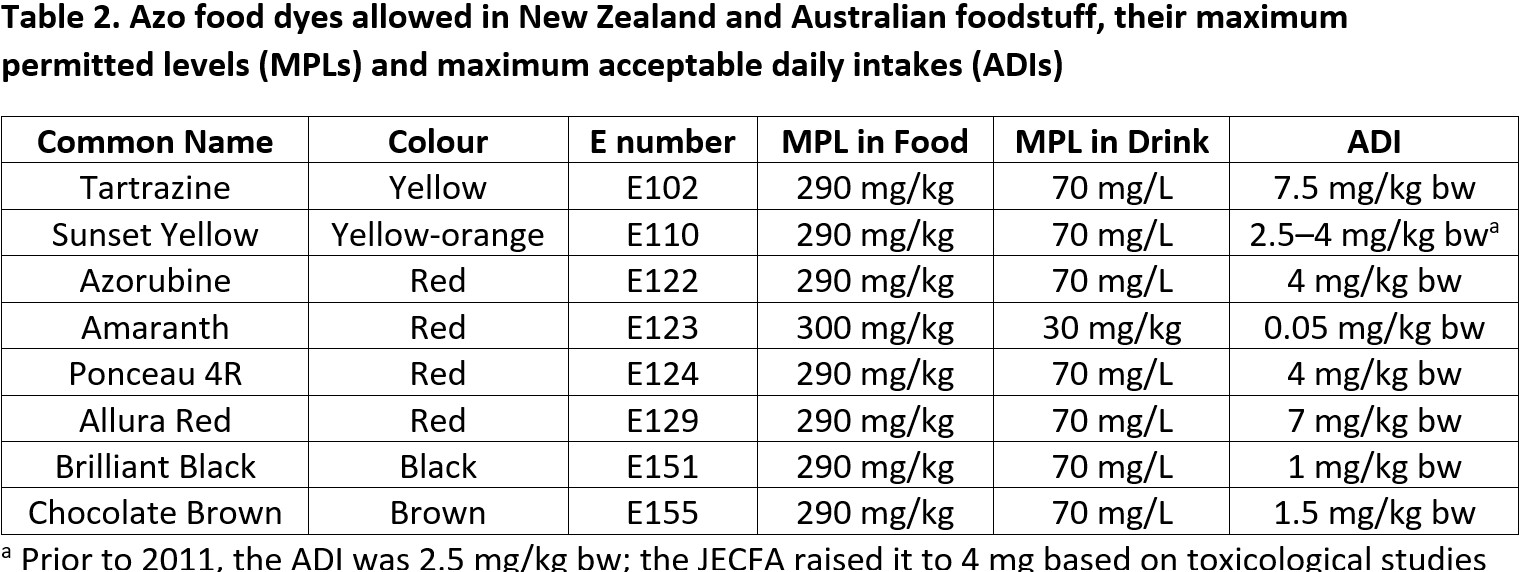
In 2008, FSANZ published a survey of food and drinks in Australia that analysed the quantities of added colours, including the eight azo dyes, in a range of food and drinks.15 It also estimated the amounts of added colourants that Australians eat based on responses from consumers about their diets. A supplementary report in 2012 accounted for updated guidelines and revisited the quantities of food dyes in diets.16 These found that nearly all foods comply with the MPL requirements for dye additives, and the vast majority are present at levels well below the MPL (with mean concentrations typically 10% or less of the MPL). In surveying consumers, the estimated quantities of each dye present in the diets of average consumers and 90th percentile consumers (those with the most processed foods in their diets) were well below the ADIs. For example, the 90th percentile consumers in the age range 6–12 years old – arguably the group most likely to be attracted to food with bright colours – were estimated to be eating a total of approximately 1 mg of allura Red, 3 mg of azorubine, 2 mg of chocolate brown, 3.8 mg of sunset yellow and 3 mg of tartrazine. Given a child in this age range is likely to be at least 20–30 kg these values are around 5% of the ADI or lower. Average consumers have much less, at ca. 1 mg or less of each dye.
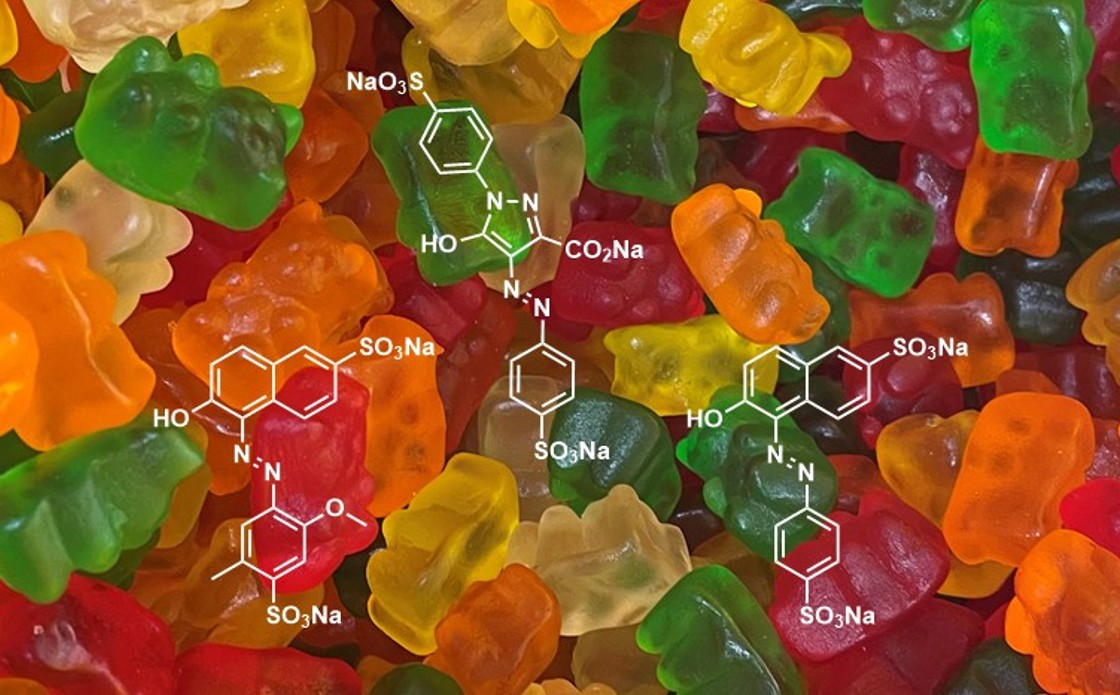
Based on my own (informal) inspections of food and drink available in New Zealand, there are a modest number of goods that contain azo dyes. Amongst chippies and other savoury snacks, only occasional brands and flavours are coloured with azo dyes (e.g. Burger Rings, Doritos Cheese Supreme). Lollies (sweets/confectionery) made in NZ are often coloured with natural dyes, although many emanating from overseas still contain intensely coloured azo dyes (e.g. M&Ms and many classic gummies) (Fig. 11). Some soft drinks (e.g. Fanta, Mountain Dew, Sparkling Duet) and sports drinks contain azo and other artificial dyes. Jelly powder and food colouring that once had only artificial dye options, are now available in brands that contain natural dyes.

Outlook
There is a growing consciousness about the effects of diet on health and interest in the science of additives.74 Given that in New Zealand all additives in food and drink must be listed on the label, those who are concerned about any adverse effects of consuming these dyes have the ability to control their consumption. It can be helpful to learn the E numbers of key additives of interest, as this is how they are typically listed. For many products, there are brand options without artificial dyes.
It is highly likely that the US move away from artificial dyes will continue the trend in New Zealand of replacing synthetic additives with natural ones. Many of the sweets and other processed foods available in New Zealand have already switched to natural colourings, such as curcumin (E100, often added as turmeric), β-carotene (E160a) and anthocyanins (E163, sometimes added as berry pulp). These colourants come from food and therefore are intrinsically edible, however it should be noted that toxicological assessments of these substances are not complete. Hence, even with natural dyes, it is recommended not to consume more of the additives than would be present in wholefoods containing them. Natural does not always mean safe!74 More research will be needed to continue to monitor and fully understand the impacts of food additives.
References
- Halpert, M. Kennedy announces ban on artificial dyes in food and drinks. BBC News, 23 April 2025. https://www.bbc.com/news/articles/cpdzyjyp8x1o (accessed 23/04/2025).
- Solis, N.; Rust, S. Newsom signs bill to expel six food dyes from California public schools. Los Angeles Times, 28 September 2024. Newsom signs bill to expel six food dyes from California schools - Los Angeles Times (accessed 23/04/2025).
- Lardieri, A. West Virginia passes ban on nine cancer-linked food ingredients in biggest shakeup of US food. Daily Mail, 26 March 2025. US's unhealthiest state passes ban on cancer-linked food ingredients (accessed 23/04/2025).
- Brown, R. Seeing red over additives. Stuff, 27 September 2010. https://www.stuff.co.nz/life-style/well-good/4169217/Seeing-red-over-additives (accessed 23/04/2025).
- Miller, M.D.; Steinmaus, C.; Golub, M.S.; Castorina, R.; Thilakartne, R.; Bradman, A.; Marty, M.A. Environmental Health 2022, 21, 45–63. https://doi.org/10.1186/s12940-022-00849-9
- Kwon, Y.H.; Banskota, S.; Wang, H.; Rossi, L.; Grondin, J.A.; Syed, S.A.; Yousefi, Y.; Schertzer, J.D.; Morrison, K.M.; Wade, M.G.; Holloway, A.C.; Surette, M.G.; Steinberg, G.R.; Khan, W.I. Nature Communications 2022, 13, 7617. https://doi.org/10.1038/s41467-022-35309-y
- Feketea, G.; Tsabouri, S. Food Chemistry 2017, 230, 578–588. https://doi.org/10.1016/j.foodchem.2017.03.043
- Vojdani, A.; Vojdani, C. Alternative Therapies in Health and Medicine 2015, 21, Suppl 1, 52–62. PMID: 25599186.
- Chung, K.T. Journal of Environmental Science and Health Part C 2016, 34, 233–261. doi:10.1080/10590501.2016.1236602 Erratum: Journal of Environmental Science and Health Part C 2017, 35, 67. https://doi.org/10.1080/10590501.2017.1284570
- McCann, D.; Barrett, A.; Cooper, A.; Crumpler, D.; Dalen, L.; Grimshaw, K.; Kitchin, E.; Lok, K.; Porteous, L.; Prince, E.; Sonuga-Barke, E.; Warner, J.O.; Stevenson, J. The Lancet 2007, 370, 1560–1567. https://doi.org/10.1016/S0140-6736(07)61306-3
- J. Meikle. FSA calls for voluntary ban on artificial colourings. The Guardian. 10 April 2008. https://www.theguardian.com/uk/2008/apr/10/foodanddrink
- Food Standards Agency. Approved additives and E numbers. https://www.food.gov.uk/business-guidance/approved-additives-and-e-numbers (accessed 29/03/2025).
- Shanker, D. Snack makers are removing fake colours from processed foods. Bloomberg, 3 March 2025. Snack Makers Are Removing Fake Colors From Processed Foods (accessed 29/03/2025).
- US Food and Drug Administration CPG Sec 587.200. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/cpg-sec-587200-uncertified-or-delisted-colors-foods-export-eg-fdc-red-2
- Survey of added colours in foods available in Australia. FSANZ, 2008. https://www.foodstandards.govt.nz/science-data/monitoring-safety/survey-of-added-colours
- Supplementary report to the 2008 Survey of added colours in foods available in Australia. FSANZ 2012. https://www.foodstandards.govt.nz/science-data/monitoring-safety/supplementary-food-colours
- Patel, S.; Rowlands, G.J. Chemistry in New Zealand 2023, October.
- Spector-Watts, B. M. Chemistry in New Zealand 2024, January.
- Khan, M.N.; Parmar, D.K.; Das, D. Mini Reviews in Medicinal Chemistry 2021, 21, 1071–1084. https://pubmed.ncbi.nlm.nih.gov/33231147/
- Josephy, P.D.; Allen-Vercoe, E. Food and Chemical Toxicology 2023, 178, 113932. https://www.sciencedirect.com/science/article/pii/S0278691523003344?via%3Dihub
- Eltaboni, F.; Bader, N.; El-Kailany, R.; Elsharif, N.; Ahmida, A. Journal of Chemical Reviews 2022, 4, 313–330. https://doi.org/10.22034/JCR.2022.349827.1177
- Alsantali, R.I.; Raja, Q.A.; Alzahrani, A.Y.A.; Sadiq, A.; Naeem, N.; Mughal, E.U.; Al-Rooqi, M.M.; El Guesmi, N.; Moussa, Z.; Ahmed, S.A. Dyes and Pigments 2022, 199, 110050. https://doi.org/10.1016/j.dyepig.2021.110050
- Barciela, P.; Perez-Vazquez, A.; Prieto, M.A. Food and Chemical Toxicology 2023, 178, 113935. https://doi.org/10.1016/j.fct.2023.113935.
- Guerra, E.; Llompart, M.; Garcia-Jares, C. Cosmetics 2018, 5, 47. https://doi.org/10.3390/cosmetics5030047
- Bafana, A.; Devi, S.S.; Chakabarti, T. Environmental Revies 2011, 19, 350–370. https://doi.org/10.1139/a11-018
- Cui, M.-H.; Liu, W.-Z.; Tang, Z.-E.; Cui, D. Water Research 2021, 203, 117512. https://doi.org/10.1016/j.watres.2021.117512.
- Morris, P.J.T.; Travis, A.S. American Dyestuff Reporter 1992, 81. https://www.researchgate.net/profile/Anthony-Travis/publication/265280328_A_History_of_the_International_Dyestuff_Industry_A_History_Of_The_International_Dyestuff_Industry/links/56e2871e08aebc9edb1b22fa/A-History-of-the-International-Dyestuff-Industry-A-History-Of-The-International-Dyestuff-Industry.pdf
- Aragaw, T.A. The Microbe 2024, 4, 100162. https://doi.org/10.1016/j.microb.2024.100162
- Wikipedia:Azo dyes. https://en.wikipedia.org/wiki/Category:Azo_dyes (accessed 31/03/2025).
- Issues and Concerns with Imported Foods. Association of Food and Drug Officials (AFDO) report, July 2019. https://www.afdo.org/wp-content/uploads/2020/11/Issues_and_Concerns_with_Imported_Foods_acc_updated_2019.pdf#%5B%7B%22num%22%3A148%2C%22gen%22%3A0%7D%2C%7B%22name%22%3A%22Fit%22%7D%5D (accessed 31/03/2025).
- PubChem: FD&C Yellow No 6. (Compound). https://pubchem.ncbi.nlm.nih.gov/compound/Sunset-Yellow-FCF#section=Methods-of-Manufacturing, (accessed 31/03/2025).
- Patel, R.; Suresh, S. Journal of Hazardardous Materials 2006, B137, 1729–1741. https://doi.org/10.1016/j.jhazmat.2006.05.019
- Solís, M.; Solís, A.; Pérez, H.I.; Manjarrez, N.; Flores, M. Process Biochemistry 2012, 47, 1723–1748. https://doi.org/10.1016/j.procbio.2012.08.014
- Sisley, P.; Porcher, C. 1911. Comptes Rendus de l’Académie des Sciences 1911, 152, 1062–1065.
- Westöö, G. 1965. Acta Chemica Scandinavica 1965, 19, 1309–1316. https://doi.org/10.3891/acta.chem.scand.19-1309
- Voyksner, R.D.; Straub, R.; Keever, J.T.; Freeman, H.S.; Hsu, W.-N. Environmental Science and Technology 1993, 27, 1665–1672. https://doi.org/10.1021/es00045a025
- Suzuki, H. Applied Microbiology and Biotechnology 2019, 103, 3965–3978. https://doi.org/10.1007/s00253-019-09775-2
- Braccia, D.J.; Minabou Ndjite, G.; Weiss, A.; Levy, S.; Abeysinghe, S.; Jiang, X.; Pop, M.; Hall, B. Drug Metabolism and Disposition 2023, 51, 142–153. doi: 10.1124/dmd.122.000898
- Ryan, A.; Laurieri, N.; Westwood, I.; Wang, C.-J.; Lowe, E.; Sim, E. Journal of Molecular Biology 2010, 400, 24–37. https://doi.org/10.1016/j.jmb.2010.04.023
- Bouhaik, I. S.; Meliani, M. H.; Suleiman, R. K.; Saleh, T. A. Polyhedron 2023, 245, 116648. https://doi.org/10.1016/j.poly.2023.116648
- Mandić, Z.; Nigović, B.; Šimunić, B. Electrochimica Acta 2004, 49, 607–615.
- Peng, Y.-J.; Xu, B.; Rokita, S. E. ACS Chemical Biology 2025, 20, 229–237.
- Imran, M.; Nagm, F.; Hussain, S.; Ashrat, M.; Ashrat, M.; Ahmad, Z.; Arshad, M.; Crowley, D. E. Clean – Soil, Air, Water 2016, 44, 1523–1530.
- Oliveira, J. M. S.; Poulsen, J. S.; Foresti, E.; Nielsen, J. L. Chemosphere 2023, 310, 136731. https://doi.org/10.1016/j.chemosphere.2022.136731
- Mahmood, S.; Khalid, A.; Arshad, M.; Mahmood, T.; Crowley, D.E. Critical Reviews in Biotechnology 2016, 36, 639–651, DOI: 10.3109/07388551.2015.1004518
- Elder, R.; Vancuren, S.J.; Botschner, A.J.; Josephy, P.D.; Allen-Vercoe, E. Anaerobe 2023, 83, 102783.
- Wolfson, S.J.; Hitchings, R.; Peregrina, K.; Cohen, Z.; Khan, S.; Yilmaz, T.; Malena, M.; Goluch, E.D.; Augenlicht, L.; Kelly, L. Nature Metabolism 2022, 4, 1260–1270. https://doi.org/10.1038/s42255-022-00656-z
- Chung, K.-T.; Fulk, G.E.; Egan, M. Applied and Environmental Microbiology 1978, 35, 558–562. https://doi.org/10.1128/aem.35.3.558-562.1978
- Roxon, J.J.; Ryan, A.J.; Wright, S.E. Food and Cosmetics Toxicology 1966, 4, 419–426. https://doi.org/10.1016/S0015-6264(66)80583-7
- Daniel, J.W. Toxicology and Applied Pharmacology 1962, 4, 572–594. https://doi.org/10.1016/0041-008X(62)90085-6
- Pay, R.; Sharrock, A.V.; Elder, R.; Maré, A.; Bracegirdle, J.; Torres, D.; Malone, N.; Vorster, J.; Kelly, L.; Ryan, A.; Josephy, P.D.; Allen-Vercoe, E.; Ackerley, D.F.; Keyzers, R.A.; Harvey, J.E. Food and Chemical Toxicology 2023, 182, 114193. doi: 10.1016/j.fct.2023.114193.
- Pérez-Díaz, I.M.; Kelling, R.E.; Hale, S.; Breidt, F.; McFeeters, R.F. Journal of Food Science 2007, 72, M240–M245. https://doi.org/10.1111/j.1750-3841.2007.00460.x
- Pérez-Díaz, I.M.; McFeeters R.F. Journal of Applied Microbiology 2009, 107, 584–589. https://doi.org/10.1111/j.1365-2672.2009.04227.x
- 2,7-Naphthalenedisulfonic acid, 3-hydroxy-4-[(4-sulfo-1-naphthalenyl)azo]-, compounds. Australian Industrial Chemicals Introduction Scheme, Australian Government: Department of Health, 2022. https://www.industrialchemicals.gov.au/sites/default/files/2022-06/EVA00050%20-%20Evaluation%20statement%20-%20%2030%20June%202022.pdf
- Sulfanilic acid and its sodium salt: Human health tier II assessment. IMAP group assessment report, Australian Government, 2017. https://www.industrialchemicals.gov.au/sites/default/files/Sulfanilic acid and its sodium salt_Human health tier II assessment.pdf
- Poul, M.; Jarry, G.; Ould Elhkim, M.; Poul, J.-M. Food and Chemical Toxicology 2009, 47, 443–448. https://doi.org/10.1016/j.fct.2008.11.034
- Dietrich, H.G.; Golka, K. Frontiers in Bioscience 2012, 4, 279–288. https://doi.org/10.2741/375
- Ardern, K.D. Cochrane Database of Systematic Reviews 2001, CD000460. https://doi.org/10.1002/14651858.CD000460
- Dipalma, J.R. American Family Physician 1990, 42, 1347–1350.
- Meyer, S.K.; Probert, P.M.E.; Lakey, A.F.; Axon, A.R.; Leitch, A.C.; Williams, F.M.; Jowsey, P.A.; Blain, P.G.; Kass, G.E.N.; Wright, M.C. Toxicology Letters 2017, 273, 55–68. https://doi.org/10.1016/j.toxlet.2017.03.024
- Abd-Elhakim, Y.M.; Hashem, M.M.; El-Metwally, A.E.; Anwar, A.; Abo-El-Sooud, K.; Moustafa, G.G.; A. Ali, H.A. International Immunopharmacology 2018, 63, 145–154. https://doi.org/10.1016/j.intimp.2018.08.002
- Amin, K.A.; Abdel Hameid, H.; Abd Elsttar, A.H. Food and Chemical Toxicology 2010, 48, 2994–2999. https://doi.org/10.1016/j.intimp.2018.08.002
- Gao, Y.; Li, C.; Shen, J.; Yin, H.; An, X.; Jin, H. Journal of Food Science 2011, 76, T125–129. https://doi.org/10.1111/j.1750-3841.2011.02267.x
- Chung, K.T. Journal of Environmental Science and Health Part C 2000, 18, 51–74. https://doi.org/10.1080/10590500009373515
- Ameur, F.Z.; Mehedi, N.; Kheroua, O.; Saïdi, D.; Salido, G.M.; Gonzalez, A. Food and Chemical Toxicology 2018, 120, 71–80. https://doi.org/10.1016/j.fct.2018.07.001
- Osman, M.Y.; Sharaf, I.A.; Osman, H.M.Y.; El-Khouly, Z.A.; Ahmed, E.I. British Journal of Biomedical Science 2004, 61, 128–132.
- Stevens, L.J.; Burgess, J.R.; Stochelski, M.A.; Kuczek, T. Clinical Pediatrics 2014, 53, 133–140. https://doi.org/10.1177/0009922813502849
- Stevens, L.J.; Burgess, J.R.; Stochelski, M.A.; Kuczek, T. Clinical Pediatrics 2015, 54, 309–321. https://doi.org/10.1177/0009922814530803
- Hou, K.; Wu, Z.-X.; Chen, X.-Y. Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; Chen, Z.-S. Signal Transduction and Targeted Therapy 2022, 7, 135. https://doi.org/10.1038/s41392-022-00974-4
- Afzaal, M.; Saeed, F.; Shah, Y.A.; Hussain, M.; Rabail, R.; Socol, C.T.; Hassoun, A.; Pateiro, M.; Lorenzo, J.M.; Rusu, A.V.; Aadil, R.M. Frontiers in Microbiology 2022, 13, 999001.
- Wu, L.; Xu, Y.; Lv, X.; Chang, X.; Ma, X.; Tian, X.; Shi, Li, X.X.; Kong, X. Ecotoxicology and Environmental Safety 2021, 223, 112551. https://doi.org/10.1016/j.ecoenv.2021.112551.
- Topaç, F.O.; Dindar, E.; Uçaroğlu, S.; Başkaya, H.S. Journal of Hazardous Materials 2009, 170, 1006–1013. https://doi.org/10.1016/j.jhazmat.2009.05.080.
- Food Safety and Quality, Food and Agriculture Organisation of the United Nations. https://www.fao.org/food/food-safety-quality/scientific-advice/jecfa/en/ (accessed 23/04/2025).
- Amenabar, T. What the science says about artificial food dyes. The Washington Post, 24 April 2025. https://www.washingtonpost.com/wellness/2025/04/23/artificial-synthetic-petroleum-dye-food-coloring/ (accessed 24/04/2025).
Acknowledgements
This research was supported by a “Catalyst” grant from Te Apārangi, the Royal Society of New Zealand (20-VUW-011-CSG). I’m grateful to the whole collaboration team for all their input to the project that incentivised this article. Special mentions: providing the inspiration for our investigation of tartrazine metabolite SCAP (David Josephy); purification and MS analysis of tartrazine metabolite SCAP and testing its effects on cells (Rob Keyzers, David Ackerley, Emma Allen-Vercoe and their respective lab groups), careful proofing and suggested edits of this manuscript (David Ackerley).