Introduction
The immune system is one of the most effective surveillance systems, with the remarkable ability to distinguish between chemical structures derived from the self and from foreign entities (pathogens). The immune system is comprised of a complex network of organs, cells, and molecules that work together to prevent pathogen exposure, infection, and disease.1 This protective function involves multiple layers of defence, from physical barriers (like the skin and mucous membranes) to cellular defences (such as T cells, B cells, and antibodies).1,2 These defences are commonly classified into two subsystems: the innate and adaptive immune responses. This article will provide a brief overview of how the innate immune system recognises foreign pathogens, and how vaccines can utilise these markers to elicit an adaptive (memory) immune response.
The innate immune system
The innate immune system serves as the first line of immune defence, detecting the presence of foreign entities by recognising molecular structures that are highly conserved, meaning they are common and largely unchanged across a wide range of pathogens. These conserved structures are known as pathogen-associated molecular patterns (PAMPs) and are recognised by pattern recognition receptors (PRRs) expressed by innate immune cells.3
PRRs can be located on the cell surface or within innate immune cells, and different PRRs can recognise different classes of PAMPs, such as fragments of the bacterial cell wall, or viral nucleic acids.3 Upon binding of a PAMP, PRR activation leads to the production of cytokines - proteins involved in immune signalling - and initiates inflammation and a generalised protective immune response.3 The innate immune response generally involves phagocytes (cells which engulf and digest pathogens), the release of antimicrobial molecules, and the activation of natural killer (NK) cells which kill host cells showing general signs of infection.1 Because the innate immune system responds rapidly through the recognition of broadly conserved features, the response is non-specific and is not especially tailored to the invading pathogen.1
Pathogen-associated molecular patterns (PAMPs)
For the immune system to recognise molecular structures as non-self (or pathogen-derived), it must recognise structures that are distinct from those native to the human body. Thus, PRRs have evolved to recognise a variety of chemical classes common to pathogens, such as motifs common in viral RNA, bacterial DNA (Fig. 1), and the bacterial cell wall (Fig. 2).3
For example, RNA in mammalian cells is generally single-stranded (e.g. 1, Fig. 1), whereas double-stranded RNA (dsRNA, e.g. 2) is relatively uncommon under normal physiological conditions.4 During viral infections, dsRNA (e.g. 2) can accumulate in the host cell through its production in the viral replication cycle.3,5 Therefore, as a marker of viral infection, dsRNA (e.g. 2) can be recognised by certain PRRs (including the toll-like receptor (TLR) 3).6
Other PRRs can recognise different foreign nucleic acids such as single-stranded DNA (ssDNA) molecules with specific sequence patterns, like unmethylated cytosine-phosphate-guanosine (CpG) motifs (e.g. 3, Fig. 1), common in bacterial DNA.7,8 Although DNA in mammalian cells is generally double-stranded, ssDNA can be present, but contains very few CpG motifs. When CpG motifs are present, their cytidine nucleotides are usually methylated (e.g. 4).6,7 In contrast, bacterial DNA often contains unmethylated CpG motifs (e.g. 3) which can be recognised by PRRs (such as TLR9), thereby stimulating an immune response.6
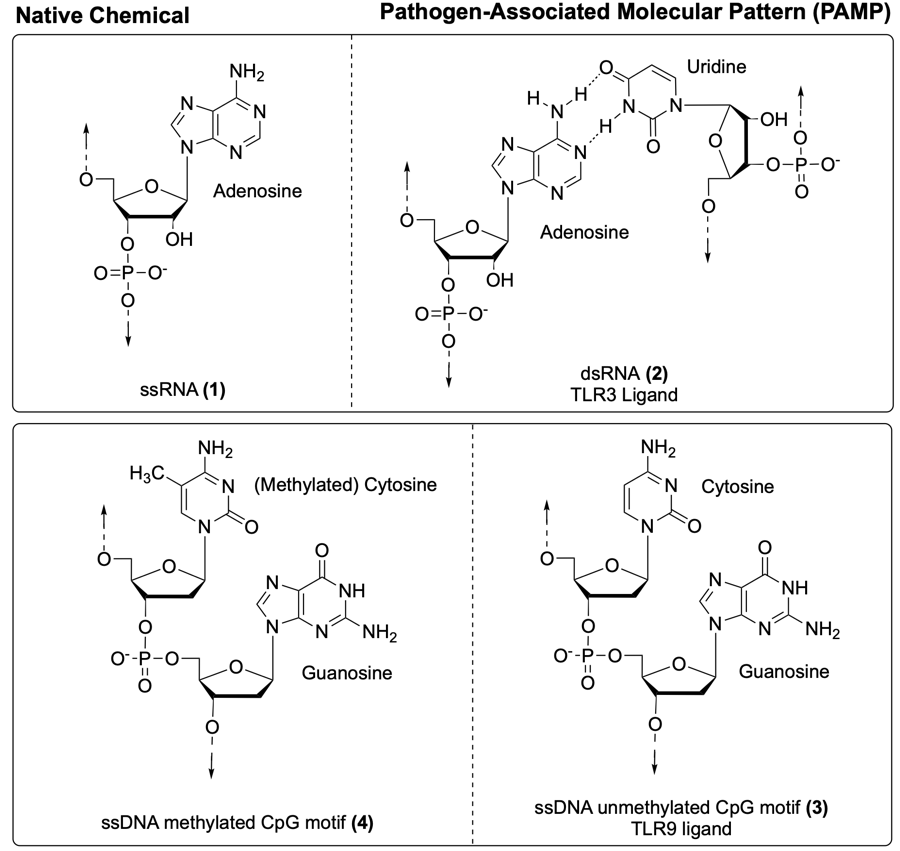
Fig. 1. Nucleic acid structures native to the human body (left) compared to nucleic acid motifs that act as pathogen-associated molecular patterns (PAMPs) (right): viral dsRNA (e.g. 2) and bacterial ssDNA with CpG motifs (e.g. 3).6
A variety of other PAMPs are associated with viral, bacterial, and fungal infections.3 For example, the bacterial cell wall - a structure absent in mammalian cells - can activate certain PRRs.3 The bacterial cell wall is a complex structure, containing a variety of immunostimulatory components (Fig. 2).
Most bacterial cell walls contain peptidoglycan, a polymer composed of polysaccharide chains with peptide crosslinks, where the minimal active structure is muramyl dipeptide (MDP, 5, Fig. 3).9 The thickness of the peptidoglycan layer typically distinguishes gram-positive bacteria (thick layer) from gram-negative bacteria (thin layer) (Fig. 2).
In the outer membrane of gram-negative bacteria, numerous glycolipids function as PAMPs including lipopolysaccharide (LPS, e.g. 6, Fig. 2), in which the Lipid A portion (e.g. 7, Fig. 3) acts as the minimal immunostimulatory structure.9 Although human cells also display glycolipids for processes such as cell signalling, the immune system is able to distinguish specific motifs that are highly conserved across bacterial species, such as Lipid A (7). The precise structure of Lipid A (7) can vary slightly between different bacterial species; however the example illustrated in Fig. 3 is derived from Escherichia coli.10
While the PAMP Lipid A (7) is recognised by TLR4, an extracellular PRR, MDP (5) is detected by NOD2 (nucleotide-binding oligomerisation domain-containing protein 2), an intracellular PRR.11 Binding of these PAMPs to their respective PRRs leads to the production of a generalised immunoprotective response.3 In addition to their role in the activation of innate immunity, PAMPs also contribute to the activation of the adaptive immune system. 3,12
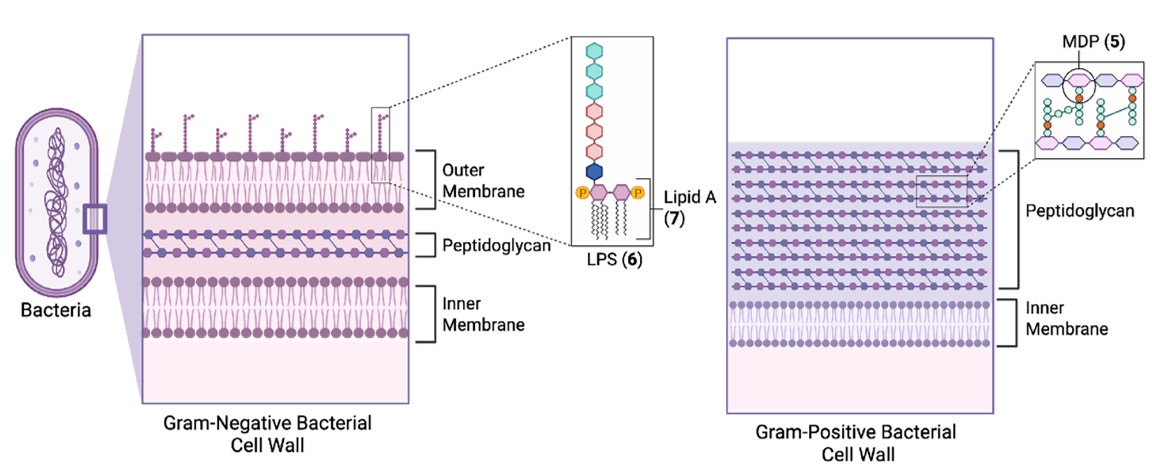
Fig. 2. Depiction of gram-negative (left) and gram-positive (right) cell walls, and immunostimulatory components LPS (6), which contains Lipid A (7), and peptidoglycan, which contains MDP (5).
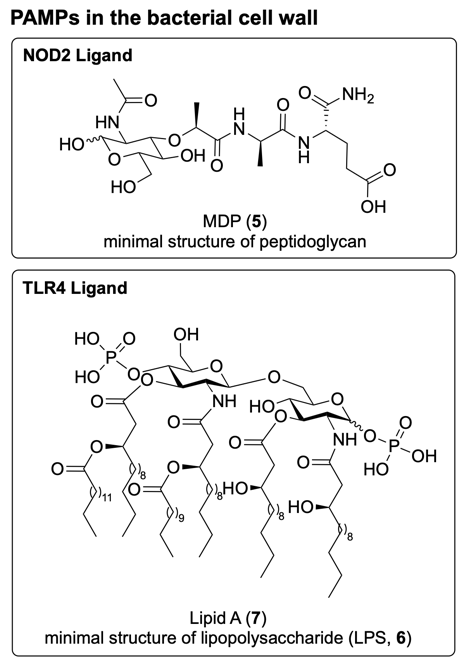
Fig. 3. Chemical structure of PAMPs present in the bacterial cell wall. MDP (5) is the minimal active structure of peptidoglycan and binds to NOD2. Lipid A (7) is the minimal active structure of lipopolysaccharide (LPS, 6), and binds to TLR4.10,11
The adaptive immune system
In contrast to the innate immune system, which recognises conserved molecular patterns, the adaptive immune system recognises structures unique to each pathogen, known as antigens.1,2 This antigen recognition enables a specialised immune response tailored to the specific pathogen. The adaptive immune response involves various cellular responses including the activation of antigen-specific B cells, which produce highly specific antibodies that neutralise the pathogen, and antigen-specific cytotoxic T cells, which can identify and kill infected host cells.2
Because the adaptive immune system recognises a specific antigen, its response can be tailored depending on the nature of the pathogen.13 For example, intracellular pathogens may trigger a stronger cytotoxic T cell response, while extracellular pathogens may prompt a more robust antibody-mediated response.
During a first encounter with a pathogen, the adaptive immune response is relatively slow to develop, requiring several days to generate an antigen-specific defence.1,2 Following this initial exposure, a subset of antigen-specific B and T cells are retained as ‘memory’ cells, known as immunological memory. Upon subsequent exposure to the same pathogen, these memory cells enable the adaptive immune system to immediately recognise the pathogen (via the antigen) and provide a specialised immune response. This rapid response can decrease the likelihood and/or severity of infection and disease.1,2
The adaptive immune response is initiated when naïve T cells, which have not yet encountered an antigen, are activated and mature into antigen-specific effector T cells.2 Effector T cells play a central role in coordinating the adaptive immune response by activating other immune cells, including antigen-specific B cells and cytotoxic T cells.1
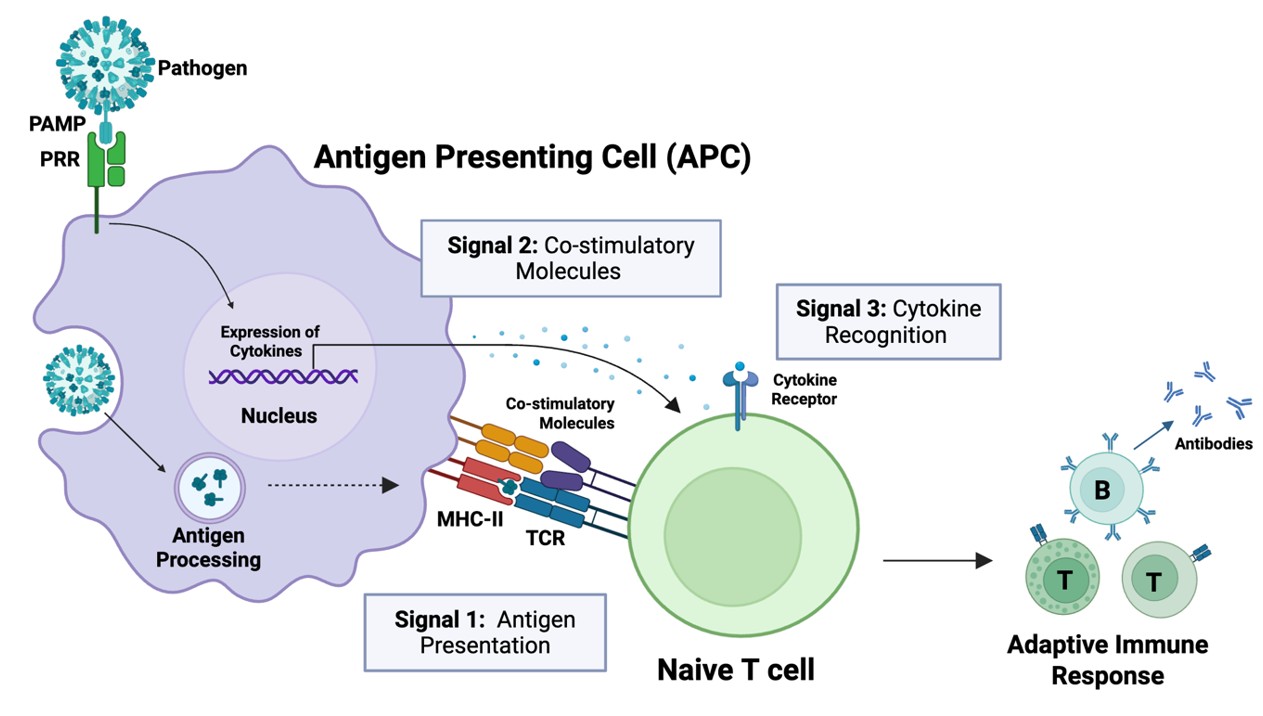
Full activation of naïve T cells requires three distinct signals to generate a robust memory response (Fig. 4). This activation is mediated by antigen-presenting cells (APCs) of the innate immune system, which display pathogen-derived antigens to naïve T cells. This process, known as antigen presentation, provides the primary activation signal (Signal 1) that drives the maturation of naïve T cells into effector T cells.2
In addition to antigen presentation, two further signals are required for full T cell activation: the binding of costimulatory molecules (Signal 2) and cytokines (Signal 3) (Fig. 4).1,2 Thus, antigen presentation alone is not sufficient for a robust memory immune response. While the antigen provides Signal 1 for T cell activation, Signals 2 and 3 are supported by PAMP-mediated activation of innate immune cells.
First, recognition of PAMPs (as well as antigens) can enhance the expression of costimulatory molecules on APCs (Signal 2), amplifying the immune response. Second, PAMP binding to APCs triggers the release of cytokines, which are recognised by naïve T cells (Signal 3). The specific cytokines released by APCs depend on the PAMP(s) that bind, and these cytokines, in turn, help determine which effector T cells develop and how they direct the adaptive immune response.1,2 Thus, both the antigen and PAMP(s) present in a pathogen influence the strength and nature of the ensuing adaptive immune response, tailoring it to the specific pathogen.1,2
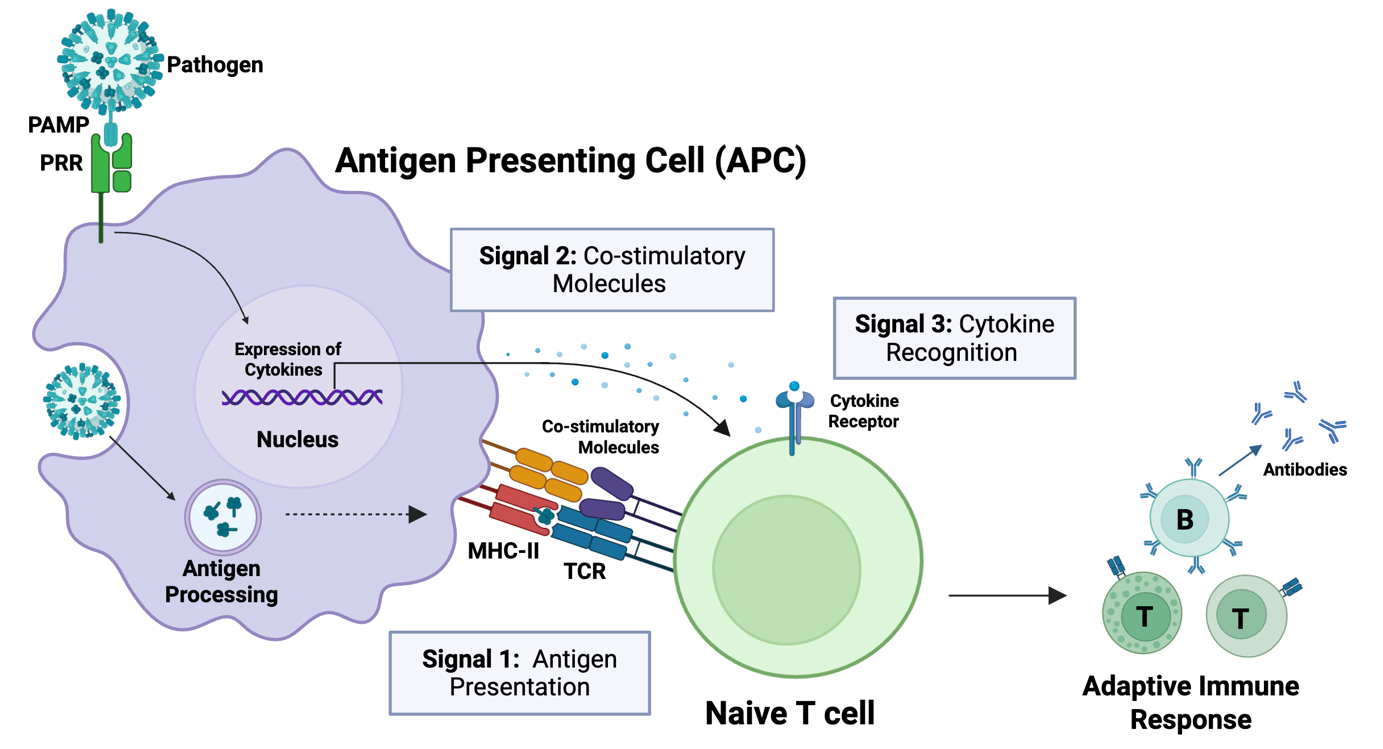
Fig. 4. Antigen-presenting cells (APCs) of the innate immune system activate the adaptive immune system through antigen presentation to naïve T cells. T cells are activated by three signals: antigen presentation (Signal 1), binding of costimulatory molecules (Signal 2), and recognition of cytokines (Signal 3).
Vaccines
Vaccines utilise the adaptive immune system to prevent infection and/or disease by serving as a controlled first exposure to a pathogen. This allows the immune system to generate immunological memory without the risk of actual infection. To elicit an effective memory response, a vaccine must provide the immune system with the appropriate antigen(s) (Signal 1) and additional immunostimulatory cues that support full T cell activation (Signals 2 & 3) for the development of long-term immunity.14
Historically, vaccines have been made with live-attenuated or inactivated (whole-killed) pathogens (Fig. 5). Such vaccines naturally provide the immune system with both the antigen and the associated inflammatory signals (PAMPs) for a robust antigen-specific memory immune response. However, many vaccines are more effective when combined with an adjuvant - an immunostimulatory component that enhances the vaccines immunogenicity (ability to stimulate an immune response).15 Appropriately, the word adjuvant is derived from the Latin verb adjuvare, meaning “to aid” or “to help”.
Adjuvants are especially important for the development of subunit and mRNA vaccines, which contain either a fragment of the whole pathogen (e.g. protein or nucleic acid sequence) or instructions to produce the antigen (in the case of mRNA vaccines) (Fig. 5).15 Because these vaccines lack the natural PAMPs present in whole pathogens, they are often poorly immunogenic when administered on their own. As such, the adjuvant helps to provide the additional immunostimulatory cues required to generate a robust memory immune response.
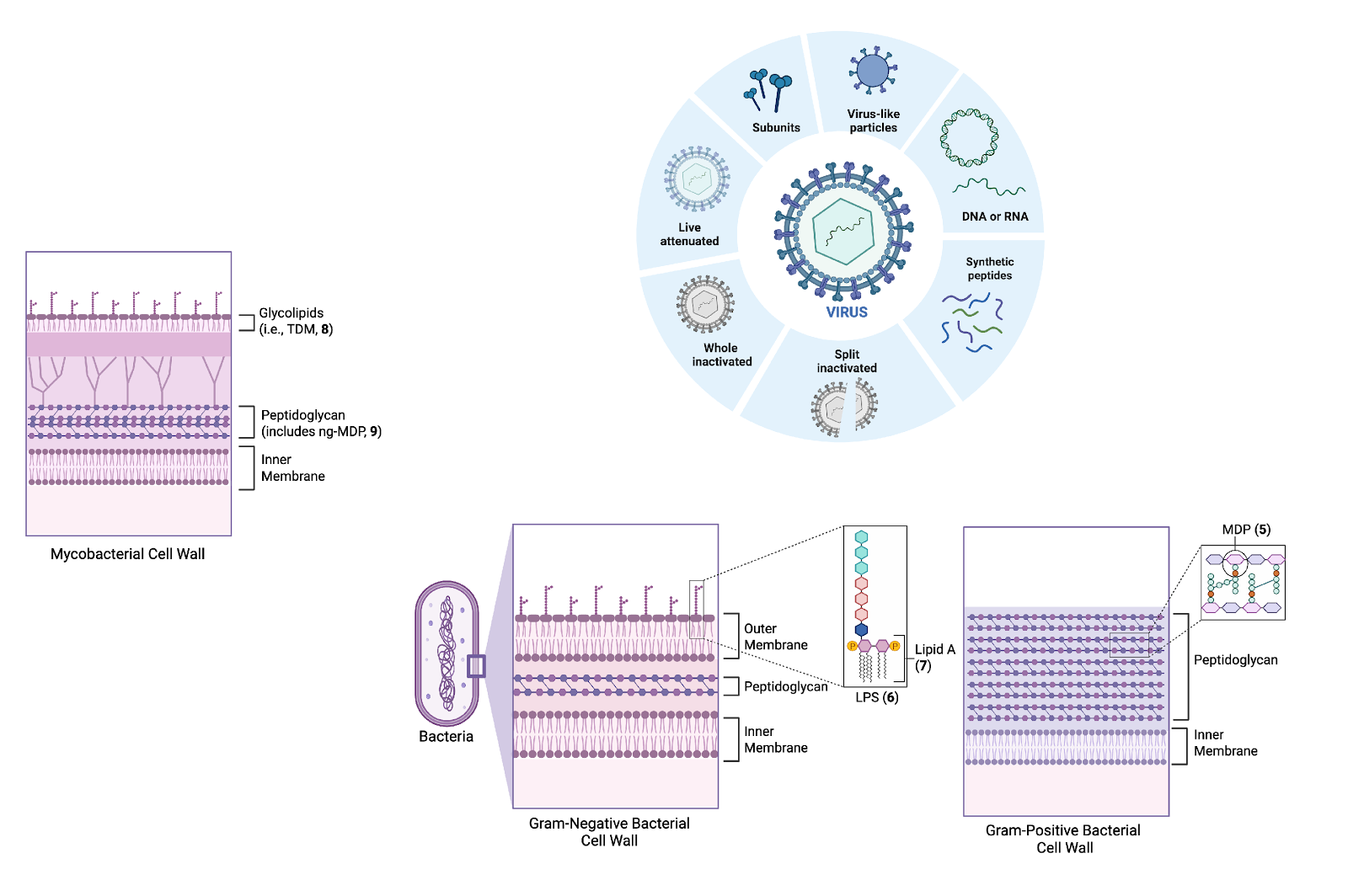
Fig. 5. Representative examples of different types of vaccines.
Vaccine adjuvants
Vaccine adjuvants can range from small molecules to complex biological extracts and inorganic salts.15 They can exert their immunogenic effects through a variety of different mechanisms, many of which are not yet fully understood.15 A common mechanism involves the activation of pattern-recognition receptors (PRRs) to generate a proinflammatory response. Some adjuvants can also function as delivery systems for the antigen, helping to prolong its bioavailability (i.e., the duration for which the antigen is available for immune recognition) and enhance its immune recognition by facilitating cellular uptake.15 Adjuvants or delivery systems that mimic the size and/or spatial organisation of natural pathogens are particularly effective, as they can be more easily recognised by the immune system and can promote antigen uptake and presentation by innate immune cells.15,16
One of the first vaccine adjuvants to be discovered – developed in the 1940s - was Freund’s Complete Adjuvant (CFA), a water-in-mineral oil emulsion containing heat-killed mycobacteria.11 This adjuvant therefore includes components of the bacterial cell wall that act as PAMPs and strongly stimulate the immune system (Fig. 6A). A major component of CFA is trehalose dimycolate (TDM, 8, Fig. 6B), a glycolipid unique to mycobacteria that binds to the extracellular PRR Mincle (Macrophage-inducible C-type lectin).11 The extract also contains the peptidoglycan fragment N-glycolyl muramyl dipeptide (ng-MDP, 9, Fig. 6B), a mycobacteria-specific variant of MDP (5, Fig. 3) that binds to the intracellular PRR NOD2.9
As a complex and variable bacterial extract, CFA has been found to cause severe inflammatory reactions in animal models, making it too toxic for widespread use in humans.9 The adjuvant effect of CFA can be largely attributed to two of the most immunogenic components: TDM (8) and ng-MDP (9).9 However, both TDM (8) and ng-MDP (9) can still induce significant toxicity when administered individually, limiting their clinical application as standalone vaccine adjuvants, as well as in CFA.9,11
Similarly, lipopolysaccharide (LPS, 6), a major component in the cell wall of gram-negative bacteria (Fig. 2), and its immunostimulatory Lipid A core (7, Fig. 3), have been studied for decades as a natural vaccine adjuvants.17 However, the toxicity and severe inflammatory responses associated with the administration of LPS (6) and Lipid A (7) have limited their use in clinical settings.17
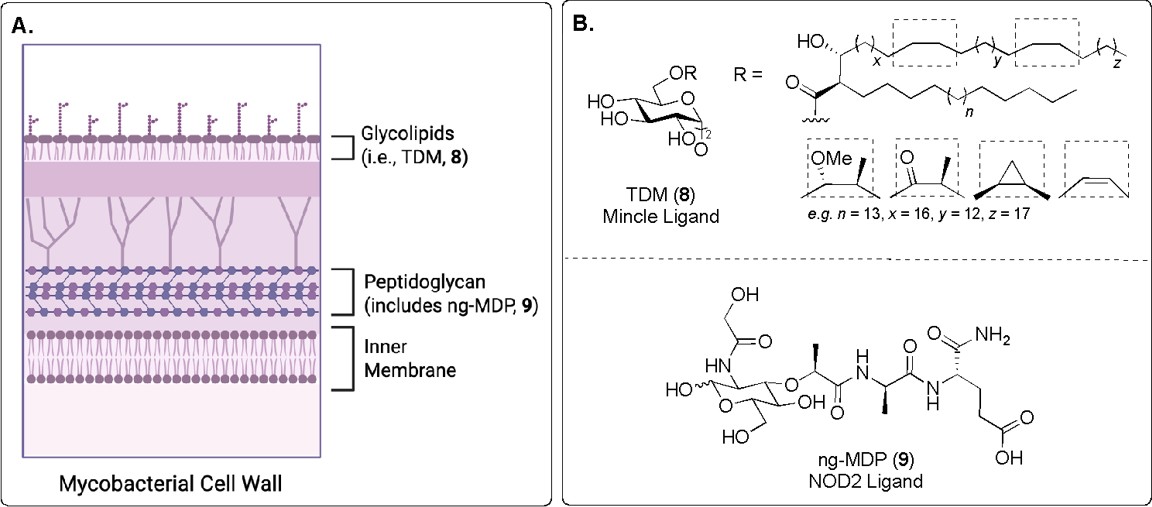
Fig. 6. A) Depiction of the mycobacterial cell wall. B) PAMPs present in Freund’s Complete Adjuvant (CFA). Trehalose dimycolate (TDM, 8) binds to the PRR Mincle, and N-glycolyl muramyl dipeptide (ng-MDP, 9) binds to the NOD2 PRR.9,11
Synthetic PAMPs as vaccine adjuvants
Due to the complex nature and inherent toxicity of bacterial extracts and natural PAMPs, synthetic PAMP mimics are increasingly favoured as vaccine adjuvants.16 These synthetic analogues can be chemically modified to reduce toxicity and improve properties such as solubility, immunostimulatory activity, and chemical stability.18
For example, Monophosphoryl Lipid A (MPL, 10, Fig. 7) is a detoxified derivative of LPS (6), maintaining the immunostimulatory core Lipid A (7), though with one fewer phosphate group.19 This chemical modification significantly reduces its toxicity compared to native LPS (6) and Lipid A (7).19 MPL (10) is now licensed for use as a vaccine adjuvant in certain contexts, including vaccines against hepatitis B virus (HBV) and human papilloma virus (HPV) in Europe and worldwide, respectively.16,20
Trehalose dibehenate (TDB, 11, Fig. 7) is a synthetic mimic of the natural glycolipid TDM (8).21 Unlike TDM (8), which is a complex and variable mixture of mycolic acids, TDB (11) is structurally well-defined and exhibits a more favourable safety profile while retaining strong adjuvant activity.11 As such, TDB (11) has been evaluated as an adjuvant in clinical trials for vaccines against Mycobacterium tuberculosis and human immunodeficiency virus (HIV). 9
Muramyl dipeptide (MDP, 5, Fig. 7), the minimal immunostimulatory component of peptidoglycan, also shows promise as a vaccine adjuvant, though it may induce toxicity and pyrogenicity (fever) at higher doses.22 To mitigate this toxicity, chemical modifications can be made to the MDP structure (5), such as the addition of lipophilic groups, to modulate its bioactivity.18,22 These lipophilic groups should either be selected to minimise disruption to NOD2 binding, or designed to be hydrolysed intracellularly, producing a hydrophilic structure capable of activating NOD2.18 One example of a safer MDP derivative is murabutide (12), where the glutamine residue of MDP (5) is alkylated with a butyl chain.23 Murabutide (12) exhibits reduced pyrogenicity compared to native MDP (5), and as a result has been used in several clinical trials as a vaccine adjuvant.18,23
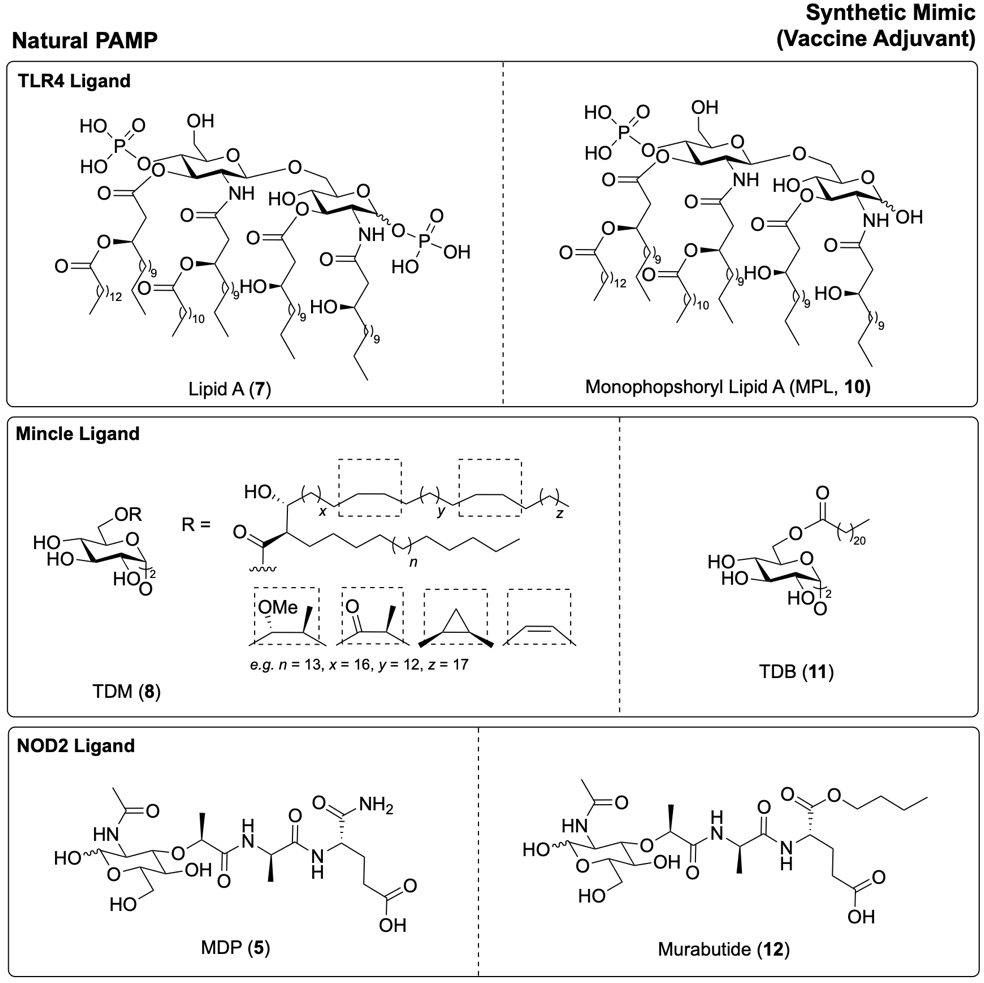
Fig. 7. TLR4 ligands Lipid A (7) and MPLA (10), Mincle ligands TDM (8)and TDB (11), and NOD2 ligands MDP (5) and murabutide (12).11,23,24
Synthetic PAMPs representing pathogen-derived nucleic acids can also serve as effective vaccine adjuvants. One example is the viral dsRNA mimic polyinosinic acid-polycytidylic acid (Poly-(I:C), 13, Fig. 8).15,25 This polymer mimics the general structure of viral dsRNA and contains inosine - a non-canonical nucleotide often produced by the deamination of adenosine in host cells (i.e. 2, Fig. 8).5 This amino-to-keto modification alters the hydrogen bonding properties of the nucleotide, favouring formation of an I:C base pair (i.e. 13).6 During viral infection, host deaminases can act on adenosine in viral dsRNA to generate inosine, destabilising the resulting I:U base pair.6 As an antiviral defence, this deamination can lead to the disruption of RNA secondary structures and/or the production of non-functional viral proteins.6 As such, inosine, and by extension the synthetic dsRNA mimic Poly-(I:C) (13), can act as viral recognition elements (PAMPs) that activate the immune system.26 In particular, Poly-I:C (13) is recognised by the PRR TLR3.4 For application as a commercial vaccine adjuvant, Poly-(I:C) (13) is much simpler to synthesise than nucleic acids with variable and more complicated sequences.4 The dsRNA mimic Poly-(I:C) (13) has now been investigated as an adjuvant in various clinical trials, for both therapeutic and cancer vaccines.11,15,25
Similarly, synthetic oligodeoxynucleotides (ODNs) containing unmethylated CpG dinucleotide motifs (e.g. 14) can mimic the immunostimulatory activity of bacterial ssDNA (e.g. 3).27 To enhance their hydrolytic stability, many CpG ODNs (e.g. 14) feature a phosphorothioate backbone instead of the natural phosphodiester linkage.27 Certain CpG ODN structures have demonstrated adjuvant activity in clinical trials, with one formulation approved for use in an HBV (hepatitis B) vaccine.16,25,28
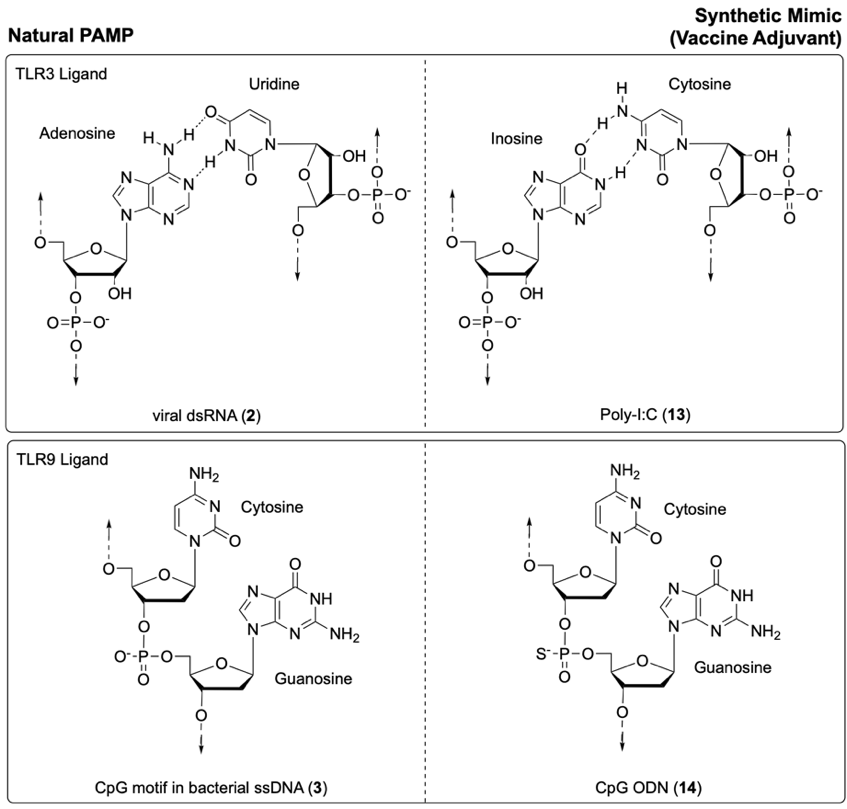
Fig. 8. Synthetic mimics of foreign nucleic acids: Poly-I:C (13) as a mimic of viral dsRNA (2), and CpG ODN (14) as a mimic of the CpG motif in bacterial ssDNA (3).6,27
Next-generation adjuvants
Adjuvants that mimic natural pathogens, whether through their size, spatial structure, or the simultaneous delivery of multiple PAMPS, are often favoured to generate a strong and long-lasting memory immune response.25 In particular, delivering multiple PAMPs to the same innate immune cell can enable cross-talk between PRR signaling pathways, the production of a unique cytokine profile, and synergistic enhancement of the immune response.22
In a recent study, Freund’s Complete Adjuvant (CFA) – which contains the highly immunogenic components TDM (8, Figure 6B) and N-glycolyl MDP (ng-MDP, 9) - was replaced with a defined two-component mixture: the synthetic TDM mimic GlcC14C18 (15, Fig. 9), and synthetic ng-MDP (9).9 As with CFA, these components were delivered in a water-in-oil emulsion for vaccination. 9 Remarkably, the synthetic PAMPs GlcC14C18 (15), and ng-MDP (9) acted synergistically to reproduce much of CFA’s adjuvant effect, but without any observed toxicity.9
Beyond co-formulation, chemically conjugating distinct PAMPs into a chimeric adjuvant can ensure their coordinated delivery to the same cell.13 One recent example is a chimeric adjuvant composed of synthetic mimics of the two most immunogenic CFA components TDM (8) and ng-MDP (9): C18Brar (16, Fig. 9), and MDP (5, Fig. 7).9,22 In this construct C18Brar-MDP (17), MDP (5) is conjugated to the lipophilic tail of C18Brar (16) to reduce its systemic toxicity.22 A pH-sensitive oxyamine linker was selected to facilitate hydrolysis and intracellular release of MDP (5), enabling it to bind to its respective PRR, NOD2.22
The dual-acting chimeric adjuvant 17 – targeting both Mincle (via C18 Brar (16)) and NOD2 (via MDP (5)) - demonstrated a synergistic effect; at low doses, the chimeric adjuvant led to a significantly stronger T cell-mediated immune response than the simple co-administration of the two components at an equivalent dose.22 This strategy highlights how rational adjuvant design can exploit immune pathway synergies to enhance the efficacy of vaccines.13
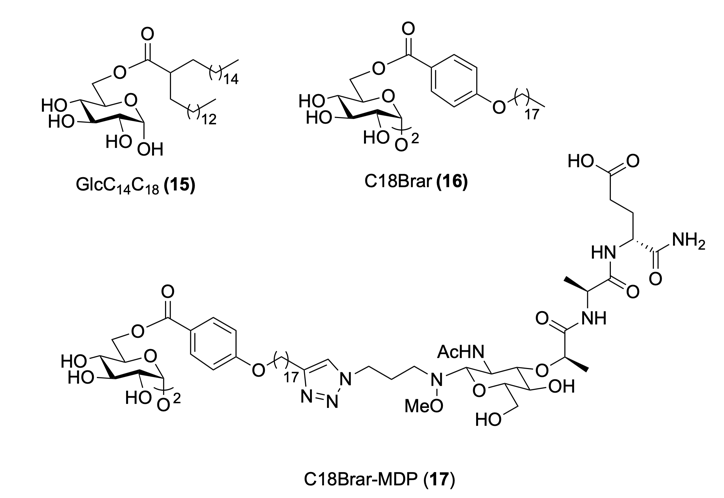
Fig. 9. Synthetic TDM (8) mimics GlcC14C18 (15) and C18Brar (16), and synthetic Mincle-NOD2 conjugate, C18Brar-MDP (17).22
Conclusions
The immune system’s ability to detect and respond to foreign molecular patterns is fundamental in initiating an effective immune response. By understanding the specific patterns that activate the innate immune system, vaccine adjuvants can be strategically designed to mimic these structures to enhance the immune response. Adjuvants that resemble natural pathogens – whether through their size and shape, or by delivering multiple PAMPs – are particularly effective at activating the immune system to generate a strong memory immune response.13
With this in mind, a diverse library of synthetic PAMP-based adjuvants can be developed, each capable of eliciting a qualitatively distinct adaptive immune response. Such a library would enable the rational pairing of antigens with adjuvants that tailor the adaptive response to best suit the target pathogen. For instance, PAMP-based adjuvants derived from bacteria may bias the adaptive immune response toward antibacterial immunity, which is particularly effective for vaccines targeting extracellular pathogens. Alternatively, PAMP-based adjuvants derived from viruses may enhance antiviral (cytotoxic) immunity, which is preferred for vaccines targeting intracellular pathogens.
This ‘mix-and-match’ approach holds great promise for future vaccine development, particularly in pandemic settings. Here, an existing adjuvant platform could be easily adapted to include a subunit antigen, streamlining the production of effective vaccines against emerging threats.
Acknowledgements
I wish to acknowledge my doctoral supervisors Associate Professor Bridget Stocker, Associate Professor Mattie Timmer, and Dr Emma Dangerfield for their guidance, support, and expertise. All biological figures were made using BioRender.
References
- Clark, R.; Kupper, T. Old Meets New: The Interaction Between Innate and Adaptive Immunity. Journal of Investigative Dermatology 2005, 125 (4), 629–637. https://doi.org/10.1111/J.0022-202X.2005.23856.X
- Wang, R.; Lan, C.; Kamel Benlagha.; Olsen, N.; Camara, S.; Miller, H.; Kubo, M.; Heegaard, S.; Lee, P.; Yang, L.; Forsman, H.; Li, X.; Zhai, Z.; Liu, C. The Interaction of Innate Immune and Adaptive Immune System. MedComm (Beijing) 2024, 5 (10), e714. https://doi.org/10.1002/mco2.714
- Mogensen, T. H. Pathogen Recognition and Inflammatory Signaling in Innate Immune Defenses. Clin Microbiol Rev 2009, 22 (2), 240. https://doi.org/10.1128/cmr.00046-08
- Gantier, M. P.; Williams, B. R. G. The Response of Mammalian Cells to Double-Stranded RNA. Cytokine Growth Factor Rev 2007, 18 (5–6), 363. https://doi.org/10.1016/J.CYTOGFR.2007.06.016
- Hur, S. Double-Stranded RNA Sensors and Modulators in Innate Immunity. Annu Rev Immunol 2019, 37, 349–375. https://doi.org/10.1146/annurev-immunol-042718-041356
- Schlee, M.; Hartmann, G. Discriminating Self from Non-Self in Nucleic Acid Sensing. Nature Reviews Immunology 2016, 16 (9), 566–580. https://doi.org/10.1038/nri.2016.78
- Häcker, G.; Redecke, V.; Häcker, H. Activation of the Immune System by Bacterial CpG-DNA. Immunology 2002, 105 (3), 245. https://doi.org/10.1046/J.0019-2805.2001.01350.X
- Chen, Y. G.; Hur, S. Cellular Origins of DsRNA, Their Recognition and Consequences. Nature Reviews Molecular Cell Biology 2021, 23 (4), 286–301. https://doi.org/10.1038/s41580-021-00430-1
- Dubé, J. Y.; McIntosh, F.; Zarruk, J. G.; David, S.; Nigou, J.; Behr, M. A. Synthetic Mycobacterial Molecular Patterns Partially Complete Freund’s Adjuvant. Scientific Reports 2020, 10 (1), 1–14. https://doi.org/10.1038/s41598-020-62543-5
- Verpalen, E. C. J. M.; Brouwer, A. J.; Boons, G. J. Synthesis of Monophosphoryl Lipid A Using 2-Naphtylmethyl Ethers as Permanent Protecting Groups. Carbohydr Res 2020, 498, 108152. https://doi.org/10.1016/j.carres.2020.108152
- Weth, A. F.; Dangerfield, E. M.; Timmer, M.; Stocker, B. L. Recent Advances in the Development of Mincle-Targeting Vaccine Adjuvants. Vaccines 2024, 12, 1320. https://doi.org/10.3390/vaccines12121320
- Li, D.; Wu, M. Pattern Recognition Receptors in Health and Diseases. Signal Transduction and Targeted Therapy 2021, 6 (1), 1–24. https://doi.org/10.1038/s41392-021-00687-0
- Tom, J. K.; Albin, T. J.; Manna, S.; Moser, B. A.; Steinhardt, R. C.; Esser-Kahn, A. P. Applications of Immunomodulatory Immune Synergies to Adjuvant Discovery and Vaccine Development. Trends Biotechnol 2019, 37 (4), 373–388. https://doi.org/10.1016/j.tibtech.2018.10.004
- Ben-Akiva, E.; Chapman, A.; Mao, T.; Irvine, D. J. Linking Vaccine Adjuvant Mechanisms of Action to Function. Sci Immunol 2025, 10 (104). https://doi.org/10.1126/sciimmunol.ado5937.
- Zhao, T.; Cai, Y.; Jiang, Y.; He, X.; Wei, Y.; Yu, Y.; Tian, X. Vaccine Adjuvants: Mechanisms and Platforms. Signal Transduction and Targeted Therapy 2023, 8 (1), 1–24. https://doi.org/10.1038/s41392-023-01557-7.
- Pulendran, B.; S. Arunachalam, P.; O’Hagan, D. T. Emerging Concepts in the Science of Vaccine Adjuvants. Nature Reviews Drug Discovery 2021, 20 (6), 454–475. https://doi.org/10.1038/s41573-021-00163-y.
- Chilton, P. M.; Hadelq, D. M.; To, T. T.; Mitchell, T. C.; Darveau, R. P. Adjuvant Activity of Naturally Occurring Monophosphoryl Lipopolysaccharide Preparations from Mucosa-Associated Bacteria. Infect Immun 2013, 81 (9), 3317. https://doi.org/ 10.1128/iai.01150-12
- Guzelj, S.; Nabergoj, S.; Gobec, M.; Pajk, S.; Klančič, V.; Slütter, B.; Frkanec, R.; Štimac, A.; Šket, P.; Plavec, J.; Mlinarič-Raščan, I.; Jakopin, Ž. Structural Fine-Tuning of Desmuramylpeptide NOD2 Agonists Defines Their in Vivo Adjuvant Activity. J Med Chem 2021, 64 (11), 7809–7838. https://doi.org/10.1021/acs.jmedchem.1c00644
- Cluff, C. W. Monophosphoryl Lipid A (MPL) as an Adjuvant for Anti-Cancer Vaccines: Clinical Results. Adv Exp Med Biol 2010, 667, 111–123. https://doi.org/10.1007/978-1-4419-1603-7_10
- Garçon, N.; Van Mechelen, M. Recent Clinical Experience with Vaccines Using MPL- and QS-21-Containing Adjuvant Systems. Expert Rev Vaccines 2011, 10 (4), 471–486. https://doi.org/10.1586/erv.11.29
- Schweneker, K.; Gorka, O.; Schweneker, M.; Poeck, H.; Tschopp, J.; Peschel, C.; Ruland, J.; Groß, O. The Mycobacterial Cord Factor Adjuvant Analogue Trehalose-6,6’-Dibehenate (TDB) Activates the Nlrp3 Inflammasome. Immunobiology 2013, 218 (4), 664–673. https://doi.org/10.1016/j.imbio.2012.07.029.
- Dangerfield, E. M.; Ishizuka, S.; Kodar, K.; Yamasaki, S.; Timmer, M. S. M.; Stocker, B. L. Chimeric NOD2 Mincle Agonists as Vaccine Adjuvants. J Med Chem 2024, 67 (7), 5373–5390. https://doi.org/10.1021/acs.jmedchem.3c01840.
- Alonso, J. M.; Hurtrel, B.; Mazigh, D.; Chalvignac, M. A.; Mollaret, H. H. Biological Activity of a New Synthetic Muramyl Peptide Adjuvant Devoid of Pyrogenicity. Infect Immun 1982, 35 (2), 417–424. https://doi.org/10.1128/iai.35.2.417-424.1982.
- Romerio, A.; Franco, A. R.; Shadrick, M.; Shaik, M. M.; Artusa, V.; Italia, A.; Lami, F.; Demchenko, A. V.; Peri, F. Overcoming Challenges in Chemical Glycosylation to Achieve Innovative Vaccine Adjuvants Possessing Enhanced TLR4 Activity. ACS Omega 2023, 8 (39), 36412–36417. https://doi.org/10.1021/acsomega.3c05363
- Facciolà, A.; Visalli, G.; Laganà, A.; Di Pietro, A. An Overview of Vaccine Adjuvants: Current Evidence and Future Perspectives. Vaccines 2022, 10 (5), 819. https://doi.org/10.3390/vaccines10050819
- Liao, J. Y.; Thakur, S. A.; Zalinger, Z. B.; Gerrish, K. E.; Imani, F. Inosine-Containing RNA Is a Novel Innate Immune Recognition Element and Reduces RSV Infection. PLoS One 2011, 6 (10), e26463. https://doi.org/10.1371/journal.pone.0026463
- Chu, R. S.; Targoni, O. S.; Krieg, A. M.; Lehmann, P. V.; Harding, C. V. CpG Oligodeoxynucleotides Act as Adjuvants That Switch on T Helper 1 (Th1) Immunity. J Exp Med 1997, 186 (10), 1623. https://doi.org/10.1084/jem.186.10.1623
- Eng, N. F.; Bhardwaj, N.; Mulligan, R.; Diaz-Mitoma, F. The Potential of 1018 ISS Adjuvant in Hepatitis B Vaccines: HEPLISAVTM Review. Hum Vaccin Immunother 2013, 9 (8), 1661. https://doi.org/10.4161/hv.24715